P-body purification
To profile P-body contents, we adapted a fluorescence-activated sorting method29,42 using a green fluorescent protein (GFP)-LSM14A expression construct in HEK293T cells (Fig. 1a). LSM14A is an established protein component of P-bodies29, and GFP-LSM14A puncta colocalized with the P-body marker EDC4 (ref. 43) (Fig. 1b). After cell lysis, intact GFP-LSM14A particles were detectable by microscopy and were readily isolated using fluorescence-activated particle sorting (FAPS) (Fig. 1c and Extended Data Fig. 1a). By contrast, particles were undetectable in cells expressing a cytoplasmic GFP control (Fig. 1c and Extended Data Fig. 1a). To corroborate these data, we depleted DDX6 in GFP-LSM14A HEK293T cells to disrupt P-bodies19,21,28,32. Both short hairpin RNA (shRNA)-mediated suppression of DDX6 and CRISPR knockout (KO) resulted in the complete loss of GFP+ particles (Fig. 1d,e and Extended Data Fig. 1b,c). To confirm the presence of RNA in GFP-LSM14A particles, we stained lysates from GFP-LSM14A-expressing cells with SYTOX Blue, an RNA-binding fluorescent dye. We observed RNA in GFP+ particles but not in GFP− particles (Extended Data Fig. 1d). These findings confirm the robust labeling and isolation of P-bodies using GFP-LSM14A.
a, Schematic for the purification and transcriptomic profiling of P-bodies from HEK293T cells based on GFP-LSM14A expression. b, Representative IF imaging of GFP-LSM14A puncta (green), colocalizing with EDC4 puncta (red) in HEK293T cells. Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI; blue) (scale bar, 10 μm). n = 3 independent experiments. c, Representative flow cytometry plots showing gating for GFP-LSM14A+ P-bodies in HEK293T cells. SSC, side scatter. d, Representative imaging of GFP-LSM14A puncta (green) in control and DDX6-KO HEK293T cells. Nuclei were counterstained with DAPI (blue) (scale bar, 10 μm) (left). P-body number in control (n = 50 cells) and DDX6-KO (n = 50 cells) HEK293T cells (right). Unpaired two-sided Student’s t-test, median ± s.d., ****P < 0.0001. e, Representative flow cytometry plots showing gating for GFP-LSM14A+ P-bodies in control and DDX6-KO HEK293T cells. f, MA plot of RNA-seq data depicting P-body-enriched genes in red and cytoplasm-enriched genes in blue in HEK293T cells (n = 2 biological independent samples per group, P < 0.05). FC, fold change; FPKM, fragments per kilobase of transcript per million mapped reads. g, GO pathway analysis of P-body-enriched mRNA in HEK293T cells, using expressed genes as background. Enrichment was tested using two-sided Fisher’s exact test with multiple-testing correction (Benjamini–Hochbergfalse discovery rate (FDR)). Padj, adjusted P value. h, GO pathway analysis of cytoplasm-enriched mRNA in HEK293T cells, using expressed genes as background. Enrichment was tested using two-sided Fisher’s exact test with multiple-testing correction (Benjamini–Hochberg FDR). i, Representative FISH imaging of POLK RNA molecules (red) combined with imaging of GFP-LSM14A puncta (green). Nuclei were counterstained with DAPI (blue) (scale bar, 10 μm). n = 3 independent experiments. j, Quantification of POLK mRNA molecules in P-bodies based on FISH (n = 50 cells; right) and P-body sequencing (right); median ± s.d. RPKM, reads per kilobase per million mapped reads. k, Read coverage distribution over the gene body of the longest annotated isoforms for genes enriched in P-bodies or the cytoplasm (cyto) in HEK293T cells. l, PolyA tail length as determined in ref. 50 compared to P-body enrichment based on Smart-seq and snapTotal-seq (Pearson correlation test). m, Translation efficiency (TE; log2 (Ribo-seq counts/RNA-seq counts)) negatively correlates with mRNA enrichment in P-bodies in HEK293T cells (Pearson correlation test (two sided), P = 3.94 × 10−98).
We characterized RNA from purified P-bodies and corresponding cytosolic fractions using Smart-seq44 (hereafter P-body-seq). We detected 3,994 mRNAs enriched in P-bodies of HEK293T cells relative to the cytosol (Fig. 1f). Gene ontology (GO) analysis revealed that P-body-enriched mRNAs encoded regulators of RNA processing, transcription, chromatin organization and cell cycle (Fig. 1g), while cytosolic mRNAs were involved in housekeeping functions such as metabolic processes and structural components (Fig. 1h). To ensure that our analyses were not biased by polyA-directed library preparations, we performed snapTotal-seq45, an alternative low-input library preparation that uses random primers rather than oligo(dT). snapTotal-seq results were highly consistent with Smart-seq data (~70% overlap; Extended Data Fig. 1e). Moreover, we compared our dataset to P-body-enriched transcripts previously reported in HEK293T cells29 and found that 3,038 mRNAs (76%) were shared between the datasets (Extended Data Fig. 1f). To confirm P-body-seq results using an orthogonal approach, we employed single-molecule fluorescence in situ hybridization (FISH) (smFISH) in conjunction with immunofluorescence (IF)46 and verified the localization of POLK and TET2 within P-bodies (Fig. 1i,j and Extended Data Fig. 1g). In line with previous reports26,38, approximately 70% of total POLK and TET2 mRNA was localized to P-bodies determined using either smFISH or P-body-seq, demonstrating substantial transcript sequestration in P-bodies.
P-bodies have been proposed as sites for RNA decay20,21,47,48; however, P-body enrichment showed poor correlation with transcript half-life measurements in HEK293T cells49, consistent with previous reports29 (Extended Data Fig. 1h). Read distribution analysis also showed no evidence of increased truncated transcripts in P-bodies relative to the cytoplasm (Fig. 1k), in line with previous reports26,29,38. In addition, we observed poor correlation between mean polyA tail length50 and P-body enrichment (r = −0.028 in Smart-seq data and r = −0.075 in snapTotal-seq data) (Fig. 1l), indicating that P-body-localized RNAs are intact and are not preferentially deadenylated relative to cytoplasmic transcripts.
We next asked whether properties intrinsic to RNAs correlated with P-body enrichment. Transcript length had no appreciable relationship with P-body localization, but transcripts with high AU content were enriched among P-body-targeted mRNAs (Extended Data Fig. 1i). Notably, AU-rich sequences are associated with inefficient translation28,51,52. Accordingly, analysis of published ribosome profiling data obtained in HEK293T cells50 revealed reduced translation efficiency for P-body-associated transcripts (Fig. 1m). These data suggest that transcripts sequestered in P-bodies are translationally repressed compared to cytoplasmic-enriched mRNAs.
P-body contents are cell type specific
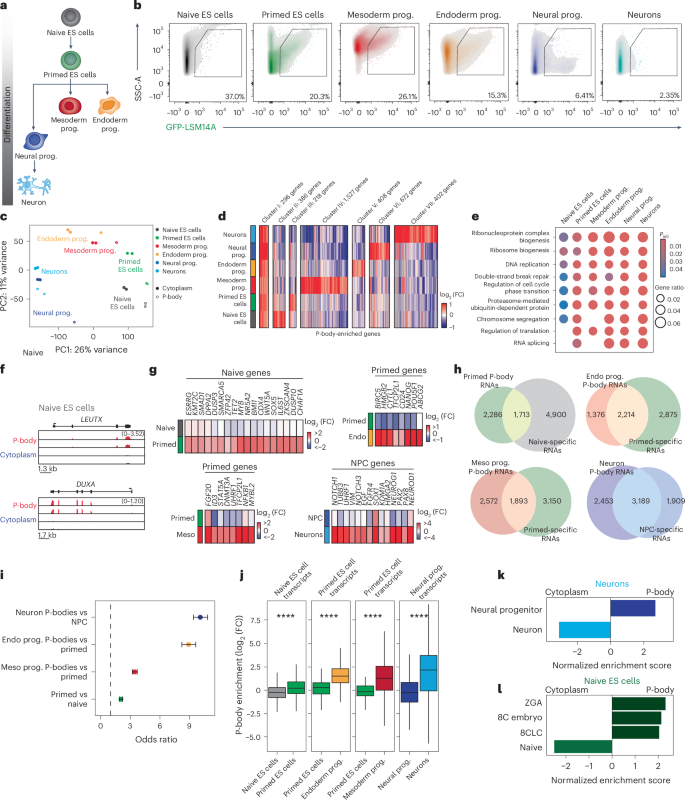
P-body-associated transcripts can reenter translation following genetic or environmental perturbation19,25,29,51,53,54, suggesting that their sequestration may influence developmental fate decisions. Yet, defining P-body function in complex biological processes has been challenging, in part because sequencing-based approaches to define P-body contents have been limited to nonvertebrates or transformed cell lines25,29,55. We thus applied P-body-seq to profile transcripts in various human cell types across developmental stages. We stably integrated GFP-LSM14A at the AAVS1 locus in human ES cells and cultured them under naive and primed conditions, which model the pre- and post-implantation epiblast, respectively56. We also induced differentiation of the same human pluripotent stem cell line into progenitors of all three germ layers (mesoderm, endoderm and neural progenitors) and ultimately into neurons (Fig. 2a). IF confirmed robust differentiation into the expected lineages (80% efficiency or greater; Extended Data Fig. 2a), which was supported by the expression of lineage-specific genes (Extended Data Fig. 2b). We detected EDC4+ P-bodies in each cell type, albeit in varying amounts (Extended Data Fig. 2c,d), suggesting differences in RNA sequestration between cell types.
a, Schematic highlighting developmental stages profiled by P-body-seq. Prog., progenitor. b, Representative flow cytometry plots showing gating for GFP-LSM14A+ P-bodies in the indicated samples. c, Principal-component (PC) analysis of RNA-seq data for the indicated samples. d, Heatmap showing expression levels of differentially enriched mRNAs between purified P-body fractions of the indicated samples, with manual clustering based on P-body-enriched genes (log2 (FC) > 0, P value < 0.05) that are specific to each cell type or enriched in all cell types (cluster I). Gene number in each cluster is indicated in the figure. Differential enrichment was assessed using DESeq2 (two-sided Wald test, Benjamini–Hochberg Padj values, P < 0.05; n = 2 biologically independent samples per group). e, GO pathway analysis using expressed genes as a background for P-body-enriched mRNA in the indicated samples. Enrichment was tested using two-sided Fisher’s exact test with multiple-testing correction (Benjamini–Hochberg FDR). f, Gene tracks showing 8C-related genes from RNA-seq data of P-bodies and cytoplasm fractions of naive ES cells. g, log2 (FC) of current cell fate markers in the current and downstream cell fate, where log2 (FC) > 0 indicates P-body enrichment and log2 (FC) < 0 indicates P-body depletion. Endo, endoderm; meso, mesoderm; NPC, neural progenitor cell. h, Venn diagrams showing the overlap of P-body-enriched genes in the downstream cell type (log2 (FC) > 0, P < 0.05) with genes that are more highly expressed in the current cell type than in the downstream cell type (current/downstream log2 (FC) > 0, P < 0.05). Differential enrichment was assessed using DESeq2 (two-sided Wald test, Benjamini–Hochberg Padj values, P < 0.05). n = 2 biologically independent samples per group. i, Odds ratio of the overlap between the comparisons in h. Center points represent the odds ratio, and error bars represent 95% confidence intervals; n of gene sets is reflected in h. Dashed line indicates an odds ratio of 1 (odds ratios >1 indicate positive association between groups). j, log2 (FC) of current fate lineage-specific genes (current/downstream log2 (FC) > 2.5, P < 0.05) in the current and downstream cell state; box plots show the median (center line), interquartile range (IQR; box, 25th–75th percentiles) and whiskers extending to 1.5× the IQR). Differential enrichment was assessed using DESeq2 (two-sided Wald test, Benjamini–Hochberg Padj values, P = 3.9 × 10−23, P = 6.4 × 10−54, P = 2.3 × 10−29, P = 1.1 × 10−37). n = 2 biologically independent samples per group (n of gene sets is reflected in h). k, GSEA performed on P-body-versus-cytoplasm differential expression in neurons for the neural progenitor-related gene expression signature and the neuron-related gene expression signature. Enrichment significance was calculated with the permutation test (two sided), with multiple-testing correction using the Benjamini–Hochberg method (FDR < 0.05). l, GSEA performed on P-body-versus-cytoplasm differential expression in human naive ES cells for human blastomere and naive ES cell-related gene sets from refs. 58,103. Enrichment significance was calculated with the permutation test (two sided), with multiple-testing correction using the Benjamini–Hochberg method (FDR < 0.05).
To profile P-body contents, we sorted GFP-LSM14A+ particles from each cell type. Particle size and fluorescence varied between cell types (Fig. 2b), likely reflecting differential expression of GFP-LSM14A from the AAVS1 locus, but, in all cases, we isolated sufficient RNA for P-body-seq. Supporting our observations in HEK293T cells, analysis of half-life data in human pluripotent stem cells57 suggested that RNA in P-bodies is not preferentially degraded compared to cytoplasmic RNA (Extended Data Fig. 2e). We observed diverse RNA biotypes in P-bodies, including protein-coding mRNA and lincRNA (Extended Data Fig. 2f). We note, however, that RNA sequestration in P-bodies is not merely a reflection of expression level, as the expression of P-body-enriched transcripts was distributed similarly relative to that of cytoplasmic and unenriched transcripts (Extended Data Fig. 2g). We likewise identified repetitive elements in P-bodies, although we did not detect marked differences in their abundance between P-bodies and the cytoplasm (Extended Data Fig. 2h,i). Based on principal-component analysis, we observed unique mRNA profiles for P-bodies from each cell type, suggesting that distinct cell identities have unique transcripts stored in P-bodies (Fig. 2c). Principal-component analysis further suggested a stepwise progression from naive ES cells toward mesoderm and/or endoderm lineages on one path and the ectoderm lineage on another (Fig. 2c). Notably, we found that P-body contents did not necessarily cluster most closely with the cytoplasm from the same cell type (Fig. 2c). For example, P-body samples from neurons cluster most closely with cytoplasmic samples from neural progenitors and P-body contents of mesoderm progenitors cluster with the cytoplasm of primed ES cells (Fig. 2c). These data suggest that P-bodies sequester transcripts from stem and progenitor cells to suppress their protein-level expression during differentiation.
P-body contents reflect preceding human developmental stages
We grouped transcripts based on their enrichment across cell types (Fig. 2d and Extended Data Fig. 2j). A fraction of transcripts was enriched in the P-bodies of all cell types, and GO categories linked to DNA damage and cell cycle progression were common across cell types (Fig. 2e). By contrast, transcripts enriched in the P-bodies of single cell types encoded fate-instructive factors, such as LEUTX and DUXA in naive ES cells (Fig. 2f). Both LEUTX and DUXA are associated with totipotency and the eight-cell (8C) embryo58,59, the developmental stage preceding naive pluripotency. To explore whether P-bodies sequester transcripts important for stem and progenitor cell maintenance during differentiation, we identified P-body-enriched transcripts related to cell fate and examined their localization in differentiated cell types and corresponding developmental precursors (Fig. 2g). P-bodies of differentiated cell types contained transcripts associated with the preceding developmental state, while these transcripts were largely cytoplasmic or unenriched in precursor cells (Fig. 2g and Discussion). smFISH analysis of OCT4 (POU5F1) in human primed ES cells and endoderm progenitors confirmed these findings, with OCT4 transcripts transitioning from primarily cytoplasmic localization in primed ES cells to P-bodies during endoderm differentiation (Extended Data Fig. 3a,b). We also observed a significant overlap between transcripts that were more expressed in precursor cells and P-body enriched in differentiated cells (Fig. 2h–j). Finally, we conducted gene set enrichment analysis (GSEA) using differential gene expression data from P-body and cytoplasmic fractions for each cell type (Fig. 2k,l and Extended Data Fig. 3c). In neurons, we observed robust enrichment of a transcriptional signature characteristic of neural progenitor cells in the P-body fraction (Fig. 2k). Consistent with these findings, P-body dissolution impaired neuronal specification from neural progenitors28,35,36, providing functional evidence that P-bodies sequester genes related to stem and progenitor cell self-renewal to facilitate differentiation. Conversely, a neuronal transcriptional signature was enriched in the cytoplasm of neurons, where these transcripts are expected to be available to translational machinery (Fig. 2k).
Next, we assessed the functional requirement of P-bodies in endoderm differentiation using a CRISPR interference (CRISPRi)-based approach to deplete DEAD-box helicase 6 (DDX6)28 and dissolve P-bodies19 (Extended Data Fig. 3d). DDX6 depletion significantly impaired differentiation toward AFP+ hepatocytes (Extended Data Fig. 3e) and suppressed the induction of other hepatic differentiation markers such as ALB, G6PC and CYP3A7 compared to control cells (Extended Data Fig. 3f). These results suggest that P-bodies are essential for differentiation to hepatocytes.
To test whether P-body-associated transcripts eventually decline after differentiation is completed, we extended neuronal differentiation to 20 d (Extended Data Fig. 3g). Analysis of P-body contents in mature neurons showed substantial overlap (~50%) with transcripts sequestered from neurons at day 7 of differentiation (Extended Data Fig. 3h); however, prolonged culture led to progressive depletion of neural progenitor-associated transcripts from P-bodies (Extended Data Fig. 3i). Similar patterns were observed in mature endoderm progenitors, which showed decreased enrichment for primed ES cell expression profiles compared to newly differentiated endoderm progenitors (Extended Data Fig. 3j,k).
We next examined P-body-associated transcripts across pluripotent states. In primed ES cells, P-bodies showed enrichment of naive ES cell-specific gene signatures (Extended Data Fig. 3c), consistent with observations that P-body dissolution promotes primed-to-naive state conversion28. Similarly, P-bodies in naive ES cells were enriched for gene expression signatures characteristic of the preceding developmental stage, including zygotic genome activation (ZGA), the 8C embryo and 8C-like cells (8CLC)58,59 (Fig. 2l and Extended Data Fig. 3l). While naive human ES cells typically express low levels of ZGA- and 8CLC-related transcripts58, our data suggest that these transcripts are sequestered into P-bodies to prevent their translation and inhibit inappropriate reversion to an earlier developmental state. Conversely, naive pluripotency transcripts were enriched in the cytoplasm relative to P-bodies (Fig. 2l), likely reflecting active expression of genes supporting the naive cell fate. These findings suggest that RNA condensates in ES cells selectively sequester totipotency-associated factors. Altogether, these data indicate that P-body contents do not simply reflect the gene expression profiles of a given cell type but are instead enriched for transcripts characteristic of the preceding developmental stage.
RNA sequestration is conserved across vertebrates
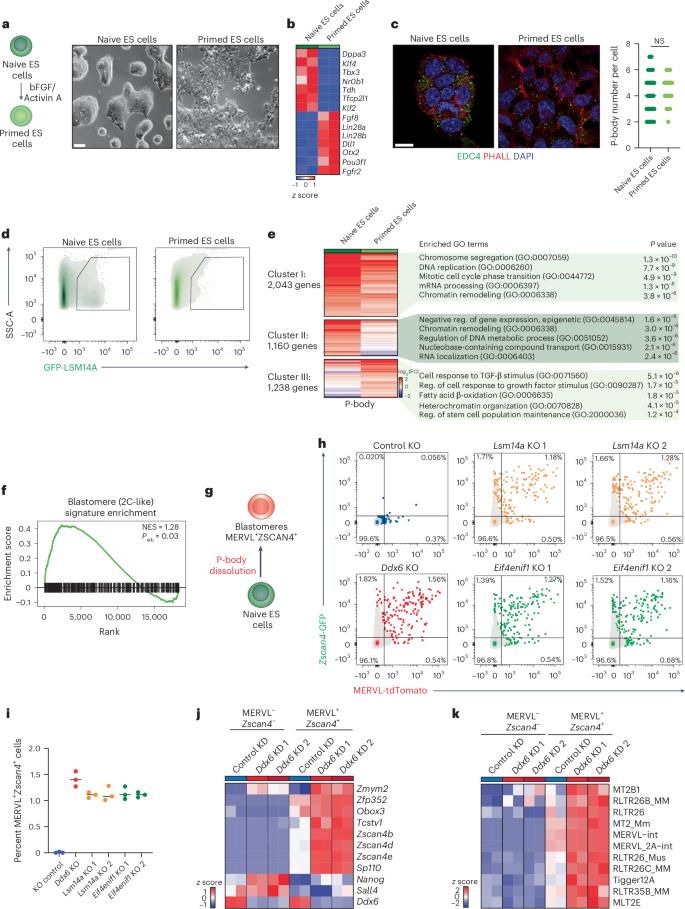
Next, we investigated whether P-body contents and functions are conserved across vertebrate species. We first considered mouse naive and primed ES cells60. Similar to their human counterparts, naive mouse ES cells resemble the in vivo pre-implantation mouse epiblast, while the primed state represents post-implantation epiblast cells61. Conversion from naive to primed mouse ES cells was efficient (Fig. 3a,b and Extended Data Fig. 4a), with both cell types harboring similar numbers of P-bodies (Fig. 3c).
a, Schematic of the conversion from naive to primed state mouse ES cells (left). Representative bright-field images of naive and primed mouse ES cells (scale bar, 50 μm) (right). n = 3 independent experiments. bFGF, basic fibroblast growth factor. b, Heatmap showing expression levels of naive-specific and primed-specific transcripts (n = 2 biological independent samples per group). c, Representative IF imaging of EDC4 puncta (green) in naive and primed mouse ES cells. Cell membranes were labeled with phalloidin (PHALL; red), and nuclei were counterstained with DAPI (blue) (scale bar, 10 μm) (left). P-body numbers in naive (n = 60 cells) and primed (n = 60 cells) mouse ES cells (right). Unpaired two-sided Student’s t-test, median ± s.d.; not significant (NS), P > 0.05, P = 0.0843. d, Representative flow cytometry plots showing gating for GFP-LSM14A+ P-bodies in naive and primed mouse ES cells. e, Heatmap showing expression levels of differentially enriched mRNAs between purified P-body fractions of naive and primed mouse ES cells, with GO pathway analysis using expressed genes as a background for P-body-enriched transcripts for the indicated clusters. Clusters were generated manually to represent genes that are P-body enriched in both naive and primed mouse ES cells or specifically enriched in one cell type. Gene number in each transcript cluster is indicated in the figure (n = 2 biological independent samples per group, P < 0.05; reg., regulation). f, GSEA analysis of blastomere-related genes63 in the purified P-body fraction versus the cytoplasmic fraction from naive mouse ES cells (normalized enrichment score (NES) = 1.28, P = 0.03). Enrichment significance was calculated using a permutation test (two sided), with multiple-testing correction using the Benjamini–Hochberg method. g, Schematic of the strategy for P-body dissolution in mouse naive ES cells carrying blastomere-specific reporters (MERVL-tdTomato and Zscan4-GFP). h,i, Flow cytometric analysis (h) and quantification (i) of MERVL-tdTomato+ and Zscan4-GFP+ cells upon Lsm14a, Ddx6 and Eif4enif1 KO in mouse naive ES cells. Control, Lsm14a, Ddx6 KO and Eif4enif1 KO (n = 3 biological independent samples per group, median ± s.d.). Tcstv1 (Tcstv1a). j,k, RNA-seq data from sorted MERVL- and Zscan4-negative and -positive mouse ES cells after Ddx6 KD, showing expression levels of pluripotency- and totipotency-related transcripts (j) and transposable elements (k).
P-body-seq analyses of mouse naive and primed ES cells (Fig. 3d) revealed that a significant fraction of P-body-enriched transcripts exhibited cell type-specific localization (54%) (Fig. 3e and Extended Data Fig. 4b), reinforcing our observations in human pluripotent stem cells. GO analysis revealed that transcripts enriched in P-bodies of both naive and primed ES cells were associated with cell cycle progression and RNA processing, similar to human datasets (Fig. 3e). Transcripts exclusively enriched in P-bodies of naive and primed ES cells were associated with chromatin remodeling and growth factor response, respectively (Fig. 3e).
Direct cross-species comparison revealed that ~50% of P-body-enriched transcripts were shared between mice and humans in both naive and primed cell states (Extended Data Fig. 4c,d).
To test whether this mechanism is conserved in phylogenetically distant vertebrates, we transduced chicken ES cells with a GFP-LSM14A lentivirus. Chicken ES cells are derived at the blastoderm stage and contribute to chimeric embryos62. In line with our findings in human and mouse cells, we detected GFP-LSM14A particles in chicken ES cells (Extended Data Fig. 4e), which we purified by FAPS. After P-body-seq, we found that mRNAs enriched in P-bodies of chicken ES cells exhibited extensive overlap with the P-body transcriptomes of mice and humans (Extended Data Fig. 4f). Comparative analysis revealed similar transcript features among all three species, particularly in the preferential sequestration of AU-rich mRNA in P-bodies, regardless of transcript length (Extended Data Fig. 4g). Thus, RNA sequestration in P-bodies is conserved across vertebrates.
Further analysis using the odds ratio statistic identified a significant positive association of chicken ES cells with both mouse and human ES cells, with the strongest association observed between mouse and human samples (Extended Data Fig. 4h). GO analysis of P-body-associated transcripts shared between chicken ES cells and mouse and human naive and primed ES cells revealed enrichment in categories related to RNA processing, transcription, chromatin organization and cell cycle (Extended Data Fig. 4i,j). These data indicate that transcripts sequestered in chicken ES cell P-bodies encode important regulatory factors, consistent with our findings in human and mouse ES cells.
Disrupting P-bodies induces translation of totipotency proteins in mouse ES cells
In human naive pluripotent stem cells, we found that P-bodies sequester transcripts related to the 8C stage embryo (Fig. 2l and Extended Data Fig. 3l). In mice, the equivalent developmental stage occurs in the two-cell embryo, although rare cells in naive mouse ES cell cultures, known as 2C cells, transiently express genes characteristic of the two-cell state63. To determine whether transcripts enriched in the P-bodies of mouse naive ES cells comprise a signature characteristic of totipotent cells, we performed GSEA. Consistent with our findings in human naive ES cells, we observed a significant enrichment of 2C-associated transcripts among P-body-enriched mRNAs, regardless of library preparation method (Fig. 3f and Extended Data Fig. 5a). smFISH analysis confirmed that the naive pluripotency markers Dppa2 and Zfp42 were predominantly cytoplasmic in naive ES cells but primarily P-body enriched in primed ES cells (Extended Data Fig. 5b–e). This observation further indicates that P-body-based regulation of developmental processes is conserved between species.
To test the functional consequence of sequestration of 2C transcripts, we employed CRISPR–Cas9 to KO Ddx6 and dissolve P-bodies in mouse ES cells harboring the 2C-specific reporters MERVL-tdTomato and Zscan4c-GFP64,65 (Fig. 3g, schematic). Ddx6 depletion significantly increased signal from both reporters (Fig. 3h,i) without altering cell proliferation (Extended Data Fig. 5f), suggesting that disrupting P-bodies facilitates the otherwise rare conversion of naive ES cells to the 2C state. RNA sequencing (RNA-seq) of MERVL and Zscan4 double-positive cells confirmed increased expression of genes (Obox3, Zscan4b, Zscan4d, Zscan4e and Zfp352) and transposable elements (MERVL, MT2B1, MLT2E) characteristic of the 2C state following Ddx6 suppression (Fig. 3j,k), suggesting that P-body dissolution enhances cell fate conversion. KO of other essential P-body factors, encoded by Eif4enif1 and Lsm14a32, similarly led to P-body loss and activation of the MERVL and Zscan4 reporters (Fig. 3h,i), supporting our hypothesis that P-bodies sequester fate-instructive 2C transcripts that are transiently suppressed and enter translation upon P-body disruption.
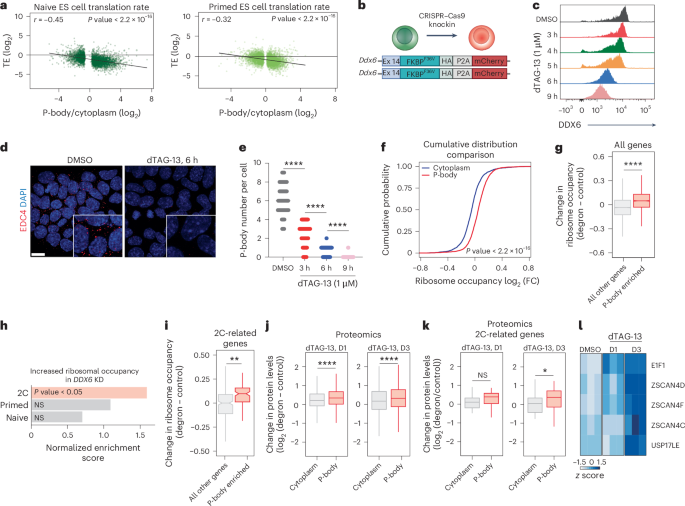
To explore this possibility further, we assessed the integrity of P-body-associated transcripts in ES cells, including those encoding 2C regulators. Analysis of both snapTotal-seq and Smart-seq data revealed over 70% overlap in P-body-associated transcripts (Extended Data Fig. 5g) between methods. Further comparison between P-body enrichment and mRNA half-lives in ES cells based on 4-thiouridine labeling66 demonstrated minimal correlation between mRNA stability and P-body enrichment (r = −0.069) (Extended Data Fig. 5h). We found no appreciable evidence of truncated mRNAs or preferential deadenylation67 of P-body-associated RNAs compared to cytosolic fractions (r = −0.092) (Extended Data Fig. 5i,j), consistent with previous reports26,29,38. These findings suggest that, in ES cells, similar to our observations in HEK293T cells, P-body-associated transcripts are not preferentially degraded compared to cytosolic transcripts. We then examined the translation rate of RNAs within P-bodies of mouse ES cells68,69. We observed a strong, inverse correlation between P-body enrichment and translation efficiency, suggesting that RNAs localized to P-bodies in naive and primed ES cells are characterized by poor translation efficiency (Fig. 4a). These results indicate that P-body-associated RNAs in ES cells are intact and translationally suppressed.
a, Translation efficiency (log2 (Ribo-seq counts/RNA-seq counts)) negatively correlates with mRNA enrichment in P-bodies in naive and primed mouse ES cells. Ribosome profiling data are from ref. 68, Pearson correlation test (two-sided). b, Schematic showing CRISPR–Cas9-based homozygous insertion of sequence for FKBP12F36V-HA-P2A-mCherry in place of the stop codon of the endogenous Ddx6 allele. Ex, exon. c, Representative intracellular flow cytometry plots for DDX6 in Ddx6-FKBP12F36V GFP-LSM14A-expressing mouse naive ES cells, either untreated (dimethylsulfoxide; DMSO) or treated with dTAG-13 at the indicated time points. d, Representative IF imaging of EDC4 puncta (red) in Ddx6-FKBP12F36V GFP-LSM14A-expressing mouse naive ES cells, either untreated (DMSO) or treated with dTAG-13 for 6 h. Nuclei were counterstained with DAPI (blue) (scale bar, 10 μm). e, P-body number in Ddx6-FKBP12F36V GFP-LSM14A-expressing mouse naive ES cells, either untreated (DMSO) or treated with dTAG-13 at the indicated time points. DMSO, n = 70 cells; 3 h, dTAG-13, n = 70 cells; 6 h, dTAG-13, n = 70 cells; 9 h, dTAG-13, n = 70 cells; unpaired two-sided Student’s t-test, median ± s.d., ****P < 0.0001. f, Cumulative distribution function plot showing ribosome occupancy (log2 (FC)) of P-body-enriched and P-body-depleted mRNAs for untreated (DMSO) versus dTAG-13-treated (6 h) Ddx6-FKBP12F36V GFP-LSM14A-expressing mouse naive ES cells; Wilcox test P = 2.97 × 10−129. g, Box plots showing the change in ribosome occupancy (log2 (ribosome-bound/total RNA) in the degron, log2 (ribosome-bound/total RNA) in the control) of P-body-enriched genes versus all other genes. Boxes indicate the IQR (25th–75th percentiles), center lines show the median, and whiskers extend to 1.5× IQR. Statistical significance was assessed using unpaired two-sided t-test (mean ± s.d., ****P < 0.0001; Padj = 1.6 × 10−38 (Holm’s method); all other genes, n = 9,133; P-body, n = 2,843). h, Normalized enrichment score of gene sets from 2C63 (P = 0.003), naive104 (P = 0.923) and primed69 (P = 0.499) samples. Enrichment significance was calculated with the permutation test (two-sided), with multiple-testing correction using the Benjamini–Hochberg method (FDR < 0.05). i, Box plots showing the change in ribosome occupancy of P-body-enriched 2C-related genes63 compared to non-P-body-enriched 2C genes. Boxes indicate the IQR (25th–75th percentiles), center show lines the median, and whiskers extend to 1.5× IQR. Unpaired two-sided t-test, mean ± s.d., **P < 0.01; Padj = 0.044 (Holm’s method); all other genes, n = 254; P-body, n = 104. j, Box plots showing the change in protein levels (log2 (degron/control)) of all P-body-enriched genes compared to P-body-depleted genes (cytoplasm) after 1 d and 3 d (D1 and D3) of dTAG-13 treatment. Boxes indicate the IQR (25th–75th percentiles), center lines show the median, and whiskers extend to 1.5× IQR. Unpaired two-sided t-test, mean ± s.d., ****P < 0.0001; Padj = 8.5 × 10−7, Padj = 6.6 × 10−6 (Holm’s method); cytoplasm, n = 1,090; P-body, n = 1,661. k, Box plots showing the change in protein levels log2 (degron/control) of P-body-enriched 2C-related genes compared to P-body-depleted genes (cytoplasm) after 1 d and 3 d of dTAG-13 treatment. Boxes indicate the IQR (25th–75th percentiles), center lines show the median, and whiskers extend to 1.5× IQR. Unpaired two-sided t-test, mean ± s.d.; not significant, P > 0.05; *P < 0.05; Padj = 0.086, Padj = 0.047 (Holm’s method); cytoplasm, n = 28; P-body, n = 42. l, Heatmap showing protein levels of 2C-related genes after 1 d and 3 d of dTAG-13 treatment compared to control samples.
To directly assess whether P-body-enriched mRNAs engage with translational machinery in stem cells after P-body dissolution, we generated a targeted DDX6 degron system in mouse naive ES cells (Ddx6-FKBP12F36V) (Fig. 4b) and performed polysome profiling after acute DDX6 loss. Addition of degradation tag-13 (dTAG-13) facilitated DDX6 protein degradation and eliminated detectable P-bodies within 6 h (Fig. 4c–e and Extended Data Fig. 6a–c). Consistent with our model, we observed a significant increase in the ribosome occupancy of P-body-associated mRNAs following DDX6 suppression compared to total cytosolic RNAs (P < 2.2−16; Fig. 4f,g). GO analysis confirmed that transcripts exhibiting enhanced ribosome occupancy were associated with stem cell maintenance (Extended Data Fig. 6d). Given the observation that disrupting P-bodies in mouse naive ES cells facilitated conversion to the 2C state, we next asked whether 2C-related transcripts demonstrated higher ribosome occupancy after DDX6 degradation. Indeed, 2C-associated mRNAs enriched in P-bodies exhibited increased ribosome occupancy after P-body dissolution (Fig. 4h,i). These data provide further evidence that mRNAs sequestered in P-bodies engage translational machinery upon their release from P-bodies in ES cells, which in turn, alters cell fate.
To measure protein-level changes, we performed large-scale, quantitative proteomics in Ddx6 degron ES cells after dTAG-13 treatment for 1 and 3 d. P-body dissolution caused significant upregulation of proteins encoded by P-body-associated mRNAs (Fig. 4j), including regulators of 2C-like cells (Fig. 4k). Elevated levels of these proteins facilitated the transition of ES cells to a 2C-like state, as shown by the increased abundance of totipotency factors such as ZSCAN4C, ZSCAN4D, ZSCAN4F, USP17LE and EIF1 (ref. 64) (Fig. 4l). These findings demonstrate that disrupting RNA sequestration in P-bodies promotes the translation of proteins important for cell fate transitions.
In line with previous studies, a fraction of P-body-enriched RNAs underwent degradation after acute loss of DDX6 (Extended Data Fig. 6e). These downregulated mRNAs were linked to differentiation processes (Extended Data Fig. 6f), perhaps reflecting a separate mechanism for protecting transcripts needed for differentiation until appropriate signals or conditions are achieved during development70.
miRNAs direct cell type-specific sequestration of mRNAs into P-bodies
Our data suggest cell type-specific sequestration of mRNA into P-bodies, yet the underlying mechanism remained unclear. We first considered RNA modifications, focusing on N6-methyladenosine (m6A), the most abundant modification in eukaryotic mRNA71. We used a human ES cell model that permits doxycycline-inducible ablation of the m6A methyltransferase METTL3 (TET-OFF METTL3)72 (Extended Data Fig. 7a). Eight days of doxycycline treatment suppressed METTL3 expression (Extended Data Fig. 7b) and reduced global m6A levels (Extended Data Fig. 7c), while pluripotency genes remained unaffected (Extended Data Fig. 7d). IF for EDC4+ puncta revealed no change in P-body numbers upon METTL3 deletion (Extended Data Fig. 7e). We profiled P-body contents and found no significant differences in METTL3-KO cells compared to the control (85% overlap; Extended Data Fig. 7f; compare white and gray circles). Moreover, only a small fraction of P-body-enriched RNA was m6A methylated, and enrichment remained largely unchanged after METTL3 KO (Extended Data Fig. 7f). These results indicate that m6A methylation does not primarily direct RNA sequestration into P-bodies in human ES cells, consistent with findings on stress granules in mouse ES cells73.
We next explored the potential involvement of RBPs in RNA sequestration within P-bodies by mapping the P-body enrichment of transcripts targeted by 53 RBPs using published crosslinking immunoprecipitation followed by sequencing (CLIP–seq) data74 (Fig. 5a). Targets of RBPs that localize to RNA granules, including PUM2 and HNRNPU19,75, were enriched in P-bodies; however, these proteins are widely expressed and unlikely to confer cell type specificity. De novo motif analysis of P-body-enriched transcripts predicted recognition by multiple RBPs that were conserved across cell types, with no evident cell type-specific patterns (Extended Data Fig. 7g,h). GO analysis of the identified RBP targets revealed enrichment for pathways consistent with known P-body functions, including 3′ untranslated region (3′-UTR) processing, ribonucleoprotein granule assembly and mRNA stabilization (Extended Data Fig. 7i). These data suggest that RBPs are unlikely to drive cell type-specific RNA sequestration.
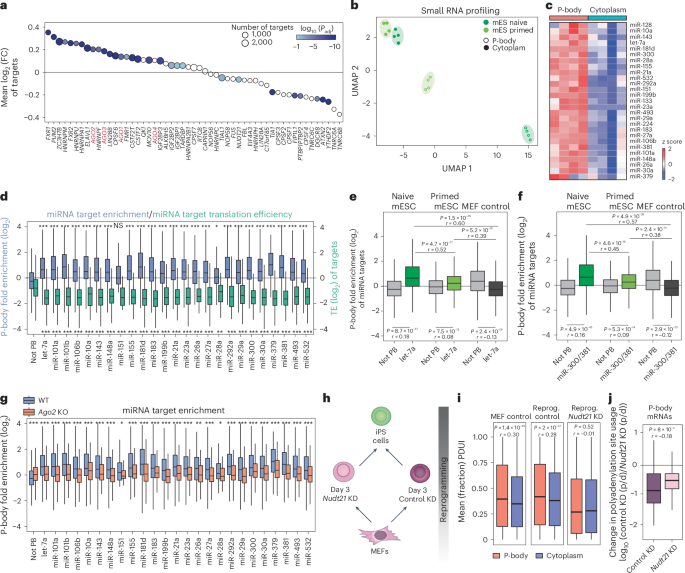
a, mRNA targets of 53 RBPs were analyzed for P-body enrichment in HEK293T cells using CLIP–seq data summarized in ref. 29. Circle size reflects the number of targets per RBP. Color indicates Benjamini–Hochberg Padj from a two-sided Wilcoxon test comparing target log2 (FC) values to those of all genes not bound by that RBP. AGO (Argonaute) protein family members are highlighted in red. C17orf85, NCBP3. PTBP1PTBP2, PTBP1 and PTBP2. b, Uniform manifold approximation and projection (UMAP) analysis of small RNA-seq data for the indicated samples based on differentially expressed genes between purified P-body and cytoplasmic fractions. mES, mouse ES cell. c, Heatmap showing expression levels of selected miRNAs in P-body and cytoplasmic fractions in mouse naive ES cells (n = 4 biological independent samples per group). d, Quantification of P-body enrichment for miRNA targets (blue) and their corresponding translation efficiency (green) from mouse naive ES cells. Ribosome profiling data are from ref. 68. Boxes indicate the IQR (25th–75th percentiles), center lines show the median, and whiskers extend to 1.5× IQR. Unpaired two-sided Wilcoxon test compares the targets of each miRNA to all genes that are not targets of P-body-enriched miRNA (PB) with Benjamini–Hochberg adjustment (*P < 0.05, **P < 0.01, ***P < 0.001; n and exact P values are noted in the source data). e,f, Box plots showing P-body enrichment of let-7a (let-7a targets, n = 395; non-P-body targets, n = 5,275) (e) and miR-300 and miR-381 (miR-300 and miR-381 targets, n = 353; non-P-body targets, n = 5,275) (f) targets in naive and primed mouse ES cells and MEFs. Unpaired Wilcoxon tests compare target genes to nontargets within each cell type (Benjamini–Hochberg Padj values and Wilcoxon r effect size reported in the figure). Boxes indicate the IQR (25th–75th percentiles), center lines show the median, and whiskers extend to 1.5× IQR. Paired, two-sided Wilcoxon tests compare enrichment between cell types (Benjamini–Hochberg Padj values and Wilcoxon r effect size are shown in the figure). g, Box plot comparing P-body enrichment of miRNA targets between WT and Ago2-KO mouse naive ES cells. Boxes indicate the IQR (25th–75th percentiles), center lines show the median, and whiskers extend to 1.5× IQR. Paired, two-sided Wilcoxon test with Benjamini–Hochberg Padj values; *P < 0.05, **P < 0.01, ***P < 0.001; NS, P > 0.05; n and exact P values are noted in the source data. h, Schematic of induced Pluripotent Stem (iPS) cell reprogramming, including perturbation of the APA regulator NUDT21. i, PolyA site usage index (PDUI) of P-body- and cytoplasm-enriched transcripts. PDUI < 0.5 indicates proximal polyA preference; PDUI > 0.5 indicates distal polyA preference. Boxes indicate the IQR (25th–75th percentiles), center lines show the median, and whiskers extend to 1.5× IQR. Two-sided Wilcoxon test, Benjamini–Hochberg Padj values and Wilcoxon r effect size are reported in the figure (MEF control, n = 1,967; MEF reprogrammed control (reprog. control), n = 2,065; Nudt21-KD reprogrammed MEF (reprog. Nudt21 KD), n = 2,189). j, Change in polyadenylation site usage after Nudt21 KO from Poly(A) Site sequencing (PAS-seq) data for genes that are lost from Nudt21-KO P-bodies compared to genes in the P-bodies of Nudt21-KO and control KO cells. p, proximal; d, distal. Boxes indicate the IQR (25th–75th percentiles), center lines show the median, and whiskers extend to 1.5× IQR. Two-sided Wilcoxon test, Benjamini–Hochberg Padj values and Wilcoxon r effect size are reported in the figure. For all panels in which effect size is reported, r < 0.1 is a negligible effect, r = 0.1–0.3 is small, r = 0.3–0.5 is medium and r > 0.5 is a large effect (control KD, n = 124; Nud21 KD, n = 404).
Further inspection of the CLIP–seq data revealed enrichment for targets of the Argonaute protein family (AGO1, AGO2, AGO3 and AGO4) within P-bodies (Fig. 5a). AGO proteins are essential components of the RNA-induced silencing complex and cooperate with miRNAs to suppress mRNA translation76,77. Previous studies have shown localization of AGO proteins in P-bodies and suggested a connection between certain miRNAs and mRNA recruitment to ribonucleoprotein granules in nonmammalian cells and transformed cell lines23,78,79,80. Moreover, miRNAs are expressed in a cell type-specific manner and play a prominent role in cell fate transitions81,82.
To test the role of miRNAs in directing RNA sequestration of developmental transcripts into P-bodies, we profiled small RNA populations in both P-body and cytoplasmic fractions across mouse and human pluripotent cells and neural progenitors using small noncoding RNA-seq83 (Extended Data Fig. 8a). Uniform manifold approximation and projection plot analysis revealed clustering of naive and primed mouse ES cell samples according to their cellular origin (Fig. 5b). Subsets of miRNAs were selectively enriched in P-bodies of pluripotent cells as well as progenitor cells (Fig. 5c and Extended Data Fig. 8b–d), including miR-300 and miR-let-7, which are known regulators of stem cell potency84,85. Targets of miRNAs enriched in P-bodies were also sequestered within these RNA condensates (Fig. 5d and Extended Data Fig. 8e–g) and translationally repressed (Fig. 5d). Further analysis86 revealed that P-body-targeted transcripts were enriched for specific miRNA binding sites that were absent in cytoplasmic transcripts (Extended Data Figs. 8h,i and 9a,b).
We plotted P-body enrichment for targets of miR-let-7, miR-300 and miR-381 across cell types. In both naive and primed ES cells, we observed increased enrichment of their targets in P-bodies (Fig. 5e,f). By contrast, we found no enrichment for target transcripts in mouse embryonic fibroblast (MEF) P-bodies (Fig. 5e,f). Together, our data suggest that miRNA-targeted genes are sequestered into P-bodies in a cell type-specific manner.
Disrupting miRNA function prevents RNA sequestration in P-bodies
Previous studies demonstrated the importance of AGO2 in the sequestration of let-7 targets into P-bodies in transformed cells78. To investigate the involvement of AGO2 in global RNA sequestration, we generated Ago2-KO mouse ES cells87 (Extended Data Fig. 9c). Ago2-KO cells had similar numbers of P-bodies as wild-type (WT) cells (Extended Data Fig. 9d), although P-bodies appeared modestly smaller (Extended Data Fig. 9d). We then sorted P-bodies from Ago2-KO ES cells and compared their transcriptome to WT counterparts. We found that Ago2 KO significantly reduced miRNA-targeted mRNA levels in P-bodies (Fig. 5g).
Next, we investigated whether modulation of polyA site usage (that is, alternative polyadenylation (APA)) influenced RNA sequestration into P-bodies. APA generates RNA isoforms that commonly differ in the length of their 3′ UTR88. Because miRNA target sequences are frequently found in the 3′ UTR, we reasoned that forcing proximal polyadenylation (that is, shorter 3′ UTRs) would relieve miRNA-based targeting of transcripts into P-bodies. To test this, we suppressed expression of Nudt21, encoding a component of the CFIm complex that facilitates distal polyadenylation5,89, in MEFs during reprogramming to pluripotency (Fig. 5h). We chose this context because a matched dataset of APA changes is available5, permitting direct comparison between polyA site usage and P-body enrichment. Nudt21 knockdown (KD) led to increased reprogramming efficiency (Extended Data Fig. 9e) and expression of pluripotency-related genes5 (Extended Data Fig. 9f). Similar to Ago2 KO, P-body numbers were largely unaffected by Nudt21 suppression (Extended Data Fig. 9g). P-body-seq from uninduced MEFs as well as reprogramming intermediates with and without Nudt21 silencing revealed that, in MEFs and day 3 control reprogramming cells, 3′ UTRs of P-body-targeted RNAs were longer than cytoplasmic counterparts, suggesting that regulatory sequences within 3′ UTRs mediate P-body enrichment (Fig. 5i). After Nudt21 suppression, we observed a global reduction in 3′ UTR length and the 3′ UTRs of transcripts enriched in P-bodies had similar lengths relative to those of cytoplasmic transcripts (Fig. 5i). We next used published data to compare changes in polyadenylation site usage to changes in transcript localization between P-bodies and the cytoplasm after Nudt21 KD5. Notably, transcripts enriched in P-bodies of control cells but absent from P-bodies after Nudt21 KD (that is, genes that relocated away from P-bodies) had shorter 3′ UTRs after Nudt21 suppression (Fig. 5j). Analysis of previously published polyA site usage data5 revealed that these transcripts contained significantly fewer miRNA target sites after Nudt21 KD (Extended Data Fig. 9h). These results suggest that the loss of miRNA binding sites prevents mRNA localization to P-bodies.
Disrupting miRNAs relocalizes target transcripts and influences cell identity
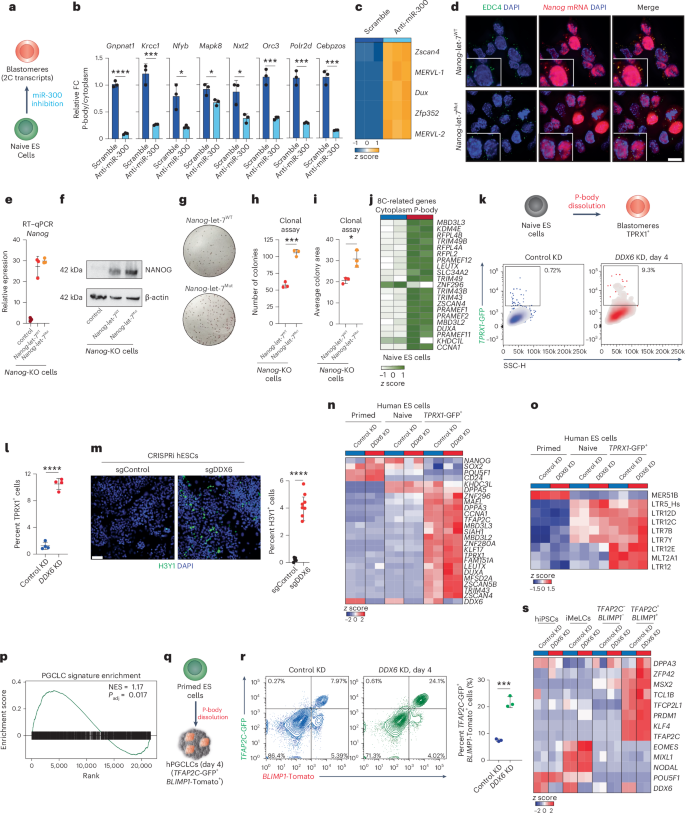
We next examined whether manipulating specific miRNAs could impact RNA localization and cell identity by depleting miR-300 in mouse ES cells using an antisense inhibitor (Fig. 6a). Following 48 h of anti-miR-300 treatment, we observed a significant reduction of miR-300 targets in P-bodies (Fig. 6b).
a, Schematic of the strategy for miR-300 inhibition in mouse naive ES cells. b, Quantitative PCR with reverse transcription (RT–qPCR) analysis of expression of miR-300 targets in P-bodies of mouse naive ES cells after miR-300 inhibition and for the control; n = 3 biological independent samples per group, unpaired two-sided Student’s t-test, mean ± s.d.; *P < 0.05, ***P < 0.001, ****P < 0.0001; P = 0.0009, P = 0.0133, P = 0.0237, P = 0.0195, P = 0.0009, P = 0.0004, P = 0.0002. c, Heatmap showing expression levels of differentially enriched 2C-related genes in mouse naive ES cells after miR-300 inhibition and for the control. d, Representative IF imaging of EDC4 puncta (green) and Nanog-MS2 (red) in Nanog-let-7WT (top) and Nanog-let-7Mut cells (bottom). Nuclei were counterstained with DAPI (blue) (scale bar, 10 μm). n = 3 independent experiments. e, RT–qPCR analysis of Nanog expression in Nanog-let-7WT and Nanog-let-7Mut cells compared to control cells (Nanog KO). n = 3 biologically independent samples per group, mean ± s.d. f, Representative western blot showing NANOG protein levels in Nanog-let-7WT and Nanog-let-7Mut cells compared to control cells (Nanog KO). g–i, Representative pictures (g) and quantification of alkaline phosphatase staining of cell colony number (h) and size (i) of Nanog-let-7WT and Nanog-let-7Mut cells cultured with fetal bovine serum and leukemia inhibitory factor (LIF). Unpaired two-sided Student’s t-test, n = 3 biologically independent samples per group, mean ± s.d.; *P < 0.05, ***P < 0.001; P = 0.0002, P = 0.0135. j, Heatmap showing expression levels of differentially enriched 8C-related mRNAs between purified P-body and cytoplasmic fractions in human naive ES cells (n = 2 biological independent samples per group). k, Schematic of the strategy for P-body dissolution in naive human ES cells carrying a blastomere-specific reporter (TPRX1-GFP). Flow cytometric analysis of TPRX1-GFP+ expression after 4 d of DDX6 KD. k, 1,000. SSC, side scatter. l, Quantification of TPRX1-GFP+ cells upon DDX6 KD. Unpaired two-sided Student’s t-test, n = 4 biologically independent samples per group, mean ± s.d., ****P < 0.0001. m, Representative IF imaging of H3Y1 (green)-positive cells in naive human ES cells upon DDX6 KO compared to control cells. Nuclei were counterstained with DAPI (blue) (scale bar, 50 μm) (left). Quantification of H3Y1+ cells upon DDX6 suppression. Unpaired two-sided Student’s t-test, single-guide (sg)Control (n = 5 fields), sgDDX6 (n = 8 fields), mean ± s.d., ****P < 0.0001 (right). hESC, human ES cell. n,o, RNA-seq data from primed, naive and TPRX1-positive human ES cells after DDX6 KD, showing expression levels of pluripotency- and totipotency-related transcripts (n) and transposable elements (o). p, GSEA analysis for the human PGCLC-related gene expression signature96 in P-body-versus-cytoplasmic differential expression in human primed ES cells. Enrichment significance was calculated using the permutation test (two sided), with multiple-testing correction using the Benjamini–Hochberg method. q, Schematic of human iPS cell (hiPSC)-to-PGCLC differentiation. hPGCLC, human PGCLC. r, Left: flow cytometric analysis of TFAP2C-GFP and BLIMP1-Tomato expression after 4 d of PGCLC differentiation in three-dimensional aggregates. Right: quantification of TFAP2C-GFP+ and BLIMP1-Tomato+ cells by flow cytometry with three experiments. Error bars indicate mean ± s.d., n = 3 biologically independent samples per group; statistical significance was determined using a two-sided unpaired Student’s t-test; ***P < 0.001, P = 0.0003. s, RNA-seq analysis of hiPSCs (BTAG cell line96), intermediate mesenchymal-like cells (iMeLCs) and TFAP2C-and-BLIMP1-negative and -positive cells after DDX6 suppression, showing expression levels of pluripotency, mesenchymal and PGC-related genes.
To test whether releasing miR-300 targets from P-bodies was sufficient to induce a change in cell fate (Fig. 6a), we examined the expression of 2C-related genes. We observed upregulation of 2C-related transcripts, including Zscan4 and MERVL, upon miR-300 suppression, suggesting that miR-300 restricts reversion of pluripotent cells to an earlier developmental fate (Fig. 6c). Notably, these transcripts are not direct targets of miR-300, suggesting that their upregulation results from a change in cell fate, rather than regulation from the miRNA itself. These findings indicate that RNA sequestration in P-bodies can be manipulated to change cell identity.
We next tested whether mRNAs could be directed into P-bodies through the addition of miRNA target sequences. We generated reporter constructs based on the pluripotency master regulator encoded by Nanog, which included a 3′ UTR with either six WT or six mutant let-7 sequences (let-7WT and let-7Mut)90 and 24 bacteriophage MS2 stem loops (Extended Data Fig. 9i). These stem loops recruit a tagged version of the MS2 coat protein (JF546 HaloTag-NLS-MCP) to permit direct, subcellular visualization of Nanog mRNA. We note that the nuclear localization signal on MCP effectively clears free MCP from the cytoplasm, strongly increasing the signal-to-noise ratio for the assay91. After transfection into Nanog-KO ES cells, Nanog-let-7WT-MS2 transcripts colocalized with the P-body marker EDC4 (Fig. 6d and Extended Data Fig. 9j), suggesting that let-7 target sequences were sufficient to direct Nanog mRNA into P-bodies. By contrast, Nanog-let-7Mut-MS2 transcripts failed to accumulate in P-bodies (Fig. 6d and Extended Data Fig. 9j). Both constructs produced similar steady-state RNA levels (Fig. 6e), excluding the possibility that the observed localization patterns arose from differences in RNA abundance. NANOG protein levels, however, were lower in cells expressing Nanog-let-7WT-MS2, supporting a nondegradative, posttranscriptional regulatory mechanism (Fig. 6f).
Finally, we investigated whether miRNA-dependent recruitment of RNAs into P-bodies could be leveraged to alter cell identity. Under naive conditions, Nanog-KO cells self-renew; however, when switched to serum–LIF ES culture medium, NANOG helps to sustain self-renewal. We therefore transitioned Nanog-KO cells expressing either Nanog-let-7WT-MS2 or Nanog-let-7Mut-MS2 from naive to standard culture conditions. Cells expressing Nanog-MS2-let-7WT had substantially lower self-renewal capacity than Nanog-let-7Mut-MS2-expressing cells, as evidenced by increased colony number and size in clonal assays (Fig. 6g–i). These data highlight the crucial role of miRNA-mediated RNA sequestration in regulating cell potency.
Manipulating P-body-mediated RNA sequestration to direct cell fate
We next sought to manipulate P-bodies to facilitate otherwise inefficient cell fate conversions from human pluripotent stem cells to rare, clinically relevant cell types. We first focused on human totipotent-like cells, which have been identified in vitro at a frequency of 0.1% in naive pluripotent cell culture58. P-bodies in naive human pluripotent stem cells sequester transcripts associated with the totipotent 8C state (Figs. 2l and 6j), likely preventing their translation and restricting naive ES cell plasticity. To test whether P-body dissolution could overcome this restriction, we generated human naive ES cells carrying a totipotency-specific reporter, TPRX1-GFP59 (Fig. 6k). DDX6 suppression increased the proportion of TPRX1-GFP+ cells by 100-fold (~0.1% to ~10%) over previous reports58 (Fig. 6k,l). As further validation, we used CRISPRi to deplete DDX6 (ref. 28), which activated H3Y1, a DUX4 target gene expressed in the 8C embryo92(Fig. 6m). Proliferation rates were unchanged between DDX6-KD and control samples (Extended Data Fig. 9k), suggesting that disrupting P-bodies increased the rate of cell fate conversion rather than cell expansion. Sorted TPRX1+ cells displayed increased expression of genes (for example, DUXA, ZSCAN5B, ZSCAN4) and transposable elements (for example, LTR12, MLT2A1) (Fig. 6n,o) associated with totipotency and the 8C state, while downregulating primed pluripotency markers such as POU5F1 and MER51B93, consistent with enhanced cell fate conversion. These findings demonstrate that P-bodies sequester totipotency-associated transcripts to suppress their expression and suggest that manipulating P-bodies could enable the induction and expansion of rare human cell types in vitro.
We next explored whether modulating P-bodies could enhance the conversion from human ES cells to PGCLCs, which are difficult to derive in vitro, share transcriptional features with totipotent stem cells94,95 and are highly relevant for reproductive medicine. Our data indicated that P-bodies in primed human ES cells sequester transcripts associated with a primordial germ cell signature96 (Fig. 6p), including FXR1, ING2, EIF4G3 and MEIOC (Extended Data Fig. 9l). We introduced shRNAs targeting DDX6 into primed human pluripotent cells carrying primordial germ cell (PGC)-specific reporters, TFAP2C-GFP and PRDM1 (BLIMP1)-Tomato96 (Fig. 6q). We induced germ cell fate and achieved a maximum conversion efficiency of less than 8% in standard conditions; however, DDX6 suppression consistently increased the formation of TFAP2C-and-BLIMP1 double-positive PGCLCs to greater than 24% (Fig. 6r). Furthermore, DDX6-depleted TFAP2C+BLIMP1+ PGCLCs exhibited elevated expression of PGC markers, including TFAP2C, PRDM1 and DPPA3 (Fig. 6s), without significant changes in cell proliferation (Extended Data Fig. 9m). These findings demonstrate that P-body dissolution facilitates the efficient programming of primed human ES cells toward the germ cell lineage. Our work further establishes a framework for using condensate biology to expand clinically relevant stem cell populations, highlighting P-body modulation as a strategy for cell fate engineering.