Optimizing chicken ES cell culture conditions
To develop culture conditions for deriving chicken ES cells, we isolated blastodermal cells from freshly laid Rhode Island Red (RIR) chicken eggs at the EGK.X stage. We then screened a panel of small molecules, growth factors and cytokines previously shown to support self-renewal in mouse, rat or human pluripotent stem cells (Supplementary Table 1). Morphologically undifferentiated ES cell colonies emerged when the culture medium was supplemented with either the Wnt/β-catenin signaling inhibitor IWR-1 or the protein kinase C (PKC) inhibitor Gö6983 (Fig. 1a). Similar effects were observed with other Wnt and PKC inhibitors (Extended Data Fig. 1a). Notably, the combination of IWR-1 and Gö6983 produced a synergistic effect in promoting self-renewal of chicken ES cells (Extended Data Fig. 1b). ES cells derived in the presence of IWR-1 and Gö6983 expressed Nanog messenger RNA (mRNA), a key pluripotency marker24,25 (Extended Data Fig. 1c). However, chicken ES cells began to differentiate on passaging, and could not be maintained beyond two passages in the presence of IWR-1, Gö6983 or both (Extended Data Fig. 1d).
a, Representative images of chicken blastodermal cells cultured in the indicated conditions for 3 days. n = 5 independent experiments. Scale bars, 50 μm. b, Representative images of chicken ES cells maintained in IWR-1 + Gö6983 + 3% yolk from unfertilized egg (Yolk/2i) at passage 35 (left panel, scale bar, 100 μm). The right panel provides a zoomed-in view of the dotted area from the left panel image (scale bar, 50 μm). n = 5 independent experiments. c, Schematic illustrating the methodology employed to identify the active component(s) within egg yolk that facilitates chicken ES cell self-renewal. The designation ‘30–80p’ refers to precipitates obtained at 30–80% saturation of ammonium sulfate. d, SDS–PAGE analysis of various fractions obtained from the ammonium sulfate precipitates. Whole and yolk plasma mixtures were within the 50 kDa to 100 kDa range. White dotted squares highlight the regions of the gels that were excised for mass-spectrometry analysis. n = 2 independent experiments. e, A representative image of RIR chicken ES cells maintained in OT/2i at passage 25 (left panel). The right panel provides a zoomed-in view of the dotted area from the image in the left panel. n = 10 independent experiments. Scale bars, 50 μm. MWCO, molecular-weight cutoff. Illustrations in c created using BioRender.com.
We observed that chicken ES cells exhibited improved growth when a larger amount of egg yolk was carried over during embryo isolation. Moreover, ES cells retained their undifferentiation state for up to 2 weeks of initial plating when the culture medium, which contained residual yolk, was left unchanged and cells were not passaged (Extended Data Fig. 1e). This observation led us to speculate that egg yolk might contain crucial component(s) for promoting chicken ES cell self-renewal. Indeed, by supplementing the culture medium with yolk in combination with IWR-1, and Gö6983 (referred to as Yolk/2i), we were able to continuously passage chicken ES cells while maintaining their undifferentiated morphology (Fig. 1b). Notably, both fertilized and unfertilized egg yolks supported chicken ES cell self-renewal equally well (Fig. 1b and Extended Data Fig. 1f). The pluripotent identity of the expanded chicken ES cells was confirmed by high expression of Nanog mRNA, which was rapidly downregulated upon withdrawal of the self-renewal cocktail (Extended Data Fig. 1g).
Next, we aimed to identify the self-renewal-promoting component(s) within the yolk using the experimental strategy outlined in Fig. 1c. Initially, we fractionated the yolk into sedimented granules and plasma. Our results indicated that the self-renewal-promoting effect was attributed to the component(s) present in the plasma rather than the granules (Extended Data Fig. 2a). We then used molecular-weight cutoff membrane filters to fractionate the plasma into three fractions: <50 kDa, 50–100 kDa and >100 kDa. Only the 50–100-kDa fraction retained the self-renewal-promoting effect (Extended Data Fig. 2a). Further fractionation of the 50–100-kDa fraction using ammonium sulfate precipitation revealed that the protein precipitates recovered at different ammonium sulfate concentrations exhibited distinct effects on maintaining chicken ES cells. Notably, the precipitates obtained at 80% saturation of ammonium sulfate (80p) exerted the strongest effect in preserving the undifferentiated state of chicken ES cells (Extended Data Fig. 2b). SDS–PAGE analysis of the 80p fraction revealed an enriched band with an approximate size of ~75 kDa (Fig. 1d). Subsequent mass-spectrometry analysis confirmed the presence of ovotransferrin in the 80p fraction, which was identified as the protein responsible for the self-renewal-promoting effect (Extended Data Fig. 2c and Supplementary Table 2). Note that minor factors observed in the 80% fraction, such as vitellogenin, serum albumin and ovomucoid, were present at high levels in the 50% and 70% fractions, as indicated by the trend box (Extended Data Fig. 2c) and Supplementary Table 2. Neither of these fractions supported self-renewal (Extended Data Fig. 2b).
Furthermore, we successfully derived and maintained chicken ES cells from multiple breeds in the presence of ovotransferrin, IWR-1 and Gö6983 (hereafter referred to as OT/2i) (Fig. 1e and Extended Data Fig. 2d). These findings indicate that ovotransferrin is the primary self-renewal-promoting component in egg yolk. The function of ovotransferrin in the OT/2i condition could not be substituted by its mammalian ortholog, highlighting a species-specific role for ovotransferrin in supporting chicken ES cell self-renewal and survival16,26,27 (Extended Data Fig. 2e,f). Chicken ES cells cultured in OT/2i retained alkaline phosphatase activity, although at a lower intensity than that observed in mouse ES cells (Extended Data Fig. 2g). Notably, the withdrawal of either IWR-1 or Gö6983 resulted in a rapid loss of self-renewal capacity in vitro, suggesting that all components of the OT/2i cocktail are essential for the successful establishment and maintenance of chicken ES cells (Extended Data Fig. 2h).
Optimizing culture conditions for ES cells across avian species
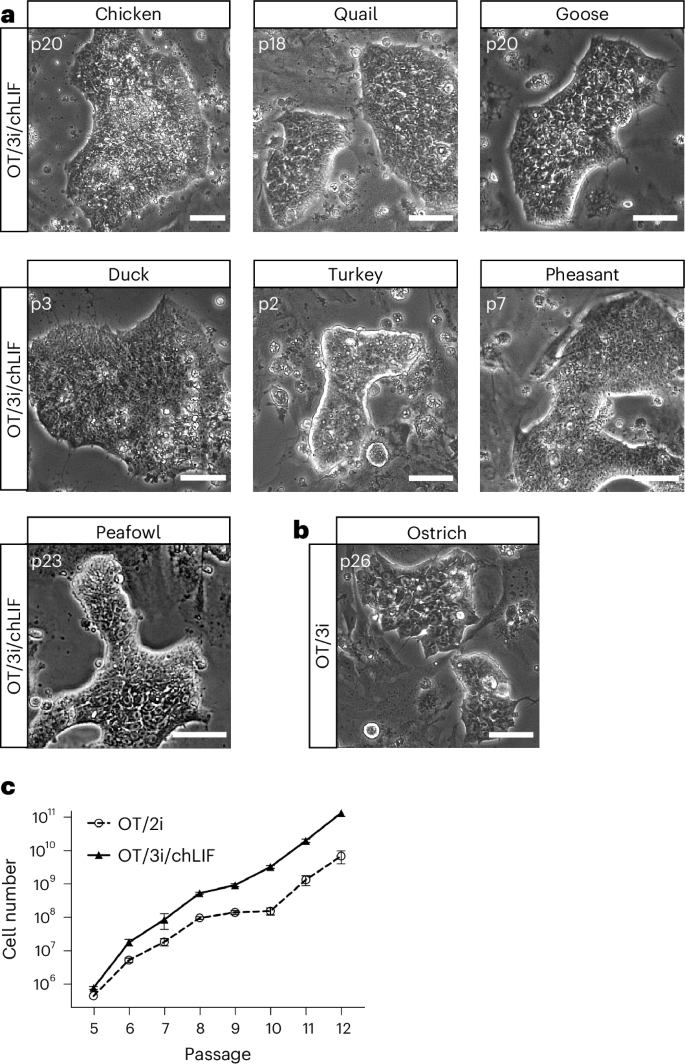
We investigated the feasibility of deriving ES cells from various avian species using the OT/2i culture system. Blastodermal cells from fertilized eggs of pheasant, duck and turkey were cultured in OT/2i. While ES cell colonies emerged within 2–3 days, they rapidly differentiated, primarily into beating cardiomyocytes (Extended Data Fig. 3a and Supplementary Video 1). SB431542, a potent and selective inhibitor of activin receptor-like kinases −4, −5 and −7, previously shown to block cardiomyocyte differentiation28, effectively prevented this differentiation and maintained pheasant, duck and turkey ES cells in an undifferentiated state (Extended Data Fig. 3b–d). However, long-term self-renewal of quail ES cells could not be sustained using OT/2i + SB431542 (hereafter referred to as OT/3i). For quail ES cell maintenance, the addition of chicken leukemia inhibitory factor (LIF) was essential (Extended Data Fig. 3e,f). The self-renewal-promoting effect of chicken LIF on quail ES cells could not be replicated using human LIF, likely due to the limited sequence homology between avian and mammalian LIF, as previously reported29 (Extended Data Fig. 3e).
While the combination of OT/3i and chicken LIF (referred to as OT/3i/chLIF) was sufficient for the derivation and maintenance of ES cells from chicken, quail, goose, duck, turkey, pheasant and peafowl, we found that ostrich ES cells could be derived and maintained in OT/3i, and that the addition of chLIF induced differentiation (Fig. 2a,b and Extended Data Fig. 3g,h). Two ES cell lines, one from chicken and one from quail, established in OT/3i/chLIF, were subjected to karyotype analysis. G-banding revealed that both lines were predominantly diploid (50 out of 50 cells for chicken ES cells and 44 out of 48 cells for quail ES cells), with approximately 10% of the quail cells exhibiting haploid or near-haploid karyotypes (Extended Data Fig. 3i,j).
a, Representative images of ES cells derived from multiple avian species in OT/3i/chLIF. n = 3 independent experiments. Scale bars, 50 μm. b, A representative image of ostrich ES cells derived and maintained in OT/3i. n = 5 independent experiments. Scale bar, 50 μm. c, Growth curves of a representative chicken ES cell line (RIR41) cultured in OT/2i or OT/3i/chLIF. Data represent the mean ± s.d. of three biological replicates.
Next, we compared the efficiency of chicken ES cell derivation and maintenance between OT/3i/chLIF and OT/2i. Both conditions successfully facilitated single-embryo derivation, resulting in eight stable ES cell lines from nine embryos in either condition and supported long-term maintenance of chicken ES cells. Notably, chicken ES cells propagated more robustly in OT/3i/chLIF than in OT/2i, suggesting a synergistic effect of SB431542 and chicken LIF with IWR-1 and Gö6983 in promoting chicken ES cell self-renewal (Fig. 2c). It is worth noting that some established chicken ES cell lines occasionally lost their characteristic colony morphology, defined by tightly packed, uniform cells, and became prone to differentiation. This phenotype typically occurred after extended culture (>20 passages) and when cells became overly confluent before passaging (Extended Data Fig. 3k). Similar to observations in stem cell cultures from other species, high cell density may create a local microenvironment that promotes sustained MAPK (MAP-kinase) signaling via combined autocrine secretion, paracrine support and extracellular matrix stabilization30,31. To address this, we tested whether inhibition of MAPK signaling, which is not directly targeted in the OT/3i/chLIF condition, could help stabilize clonal morphology during long-term culture. We found that the addition of CP-673451, a selective inhibitor of platelet-derived growth factor receptor, effectively prevented spontaneous differentiation and stabilized long-term in vitro maintenance of chicken ES cells (Extended Data Fig. 3k). Furthermore, CP-673451 improved the clonogenic efficiency of chicken ES cells (Extended Data Fig. 3l).
In the chicken embryo, a subset of epiblast cells retains pluripotency during primitive streak formation (HH2 stage)32. To determine whether these cells could be maintained under chicken ES cell culture conditions, we isolated and cultured cells from HH2-5 embryos in OT/2i, OT/3i or OT/3i/chLIF. However, all cells underwent differentiation (Extended Data Fig. 3m), suggesting that the EGK.X stage represents a restricted developmental window for successful derivation of chicken ES cells using the described conditions.
Chicken ES cells derived in OT/3i/chLIF are pluripotent
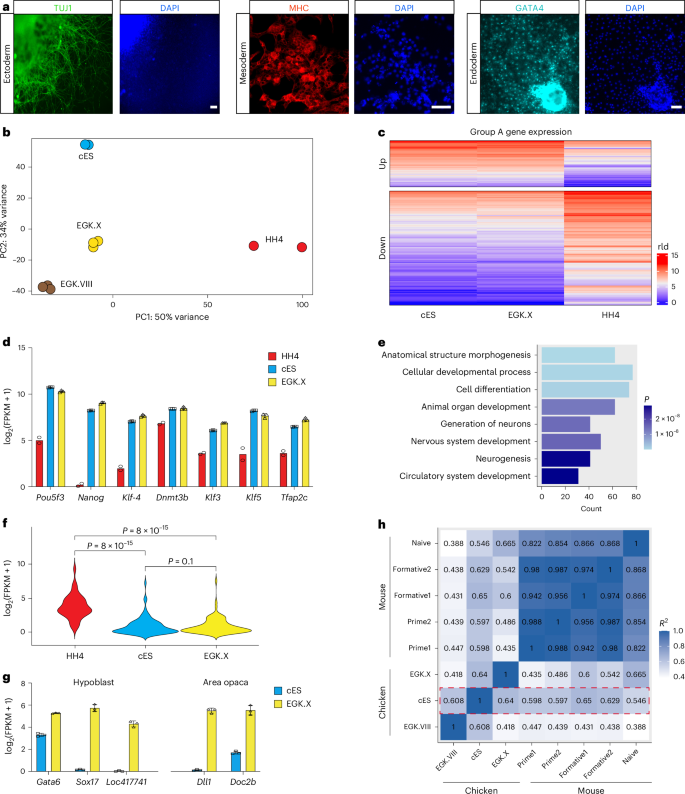
Chicken ES cells exhibit heterogeneous expression of pluripotency-associated surface markers, including stage-specific embryonic antigen-1 (SSEA-1) and epithelial membrane antigen-1, across individual colonies (Extended Data Fig. 4a). This pattern is consistent with previously reported characteristics of pluripotent stem cells in some nonmammalian systems33. To further assess the pluripotency of chicken ES cells, we induced their differentiation through embryoid body (EB) formation, a process during which ES cells spontaneously differentiate into derivatives of all three germ layers34. Quantitative PCR with reverse transcription (RT–qPCR) analysis revealed significant downregulation of the core pluripotency genes Nanog, Pou5f3 and Sall4 on EB formation24,25,35,36,37,38, along with upregulation of lineage-specific markers for ectoderm, mesoderm and endoderm (Extended Data Fig. 4b). Immunostaining further confirmed the differentiation of chicken ES cells into all three germ layers, as evidenced by the presence of TUJ1-positive (ectoderm), MHC-positive (mesoderm) and GATA4-positive (endoderm) cells (Fig. 3a). In addition, Oil Red O staining verified the differentiation of chicken ES cells into adipocytes (mesoderm) (Extended Data Fig. 4c). Together, these results demonstrate that chicken ES cells are pluripotent and possess the capacity to differentiate into all three germ layers in vitro.
a, Immunostaining of cells differentiated from chicken ES cells. n = 3 independent experiments. Scale bars, 50 μm. b, Principal component analysis (PCA) of RNA-seq data obtained from chicken ES cells (cES) and cells isolated from EGK.VIII, EGK.X and HH4 stage chicken embryos. Three biological replicates were performed for ES cells, EGK.VIII and EGK.X embryos, while two biological replicates were conducted for HH4 embryos. Data from EGK.VIII embryos were obtained from accession number GSE86592, and HH4 data were retrieved from GenBank at SRX893876. c, Heatmap analysis showing the differential gene expression profiles among chicken ES cells and cells isolated from EGK.X and HH4 stage chicken embryos. rld, regularized log transformation. d, Fragments per kilobase of transcript per million mapped reads (FPKM) values of the pluripotency genes obtained by RNA-seq analysis of chicken ES cells and cells isolated from EGK.X and HH4 stage embryos. Data represent mean ± s.d. of three biological replicates for chicken ES cells and EGK.X embryos and mean of two biological replicates for HH4 embryos. e, Gene ontology (GO) analysis of the group A downregulated genes (c) in chicken ES cells as compared to cells in HH4 embryos. Group A downregulated genes were expressed at a lower level in both chicken ES cells and EGK.X embryos compared to HH4 embryos. The significance was determined using a hypergeometric test. f, Violin plots from RNA-seq data showing the expression of differentiation-related genes (from the gene ontology term cluster). Statistical analysis was performed using the Wilcoxon test. g, FPKM values of hypoblast and area opaca markers obtained from RNA-seq analysis of chicken ES cells and cells isolated from EGK.X embryos. Error bars represent s.d. Data represent mean ± s.d. of three biological replicates. h, Transcriptome correlation analysis across species. Mouse data were from ref. 64 (GSE131556). Red dotted box highlights the comparison of chicken ES cells with various primary chicken cells or mouse pluripotent stem cells.
To further characterize the cellular identity, we conducted RNA sequencing (RNA-seq) analysis on OT/3i/chLIF chicken ES cells. Unbiased principal component analysis of bulk RNA-seq data from chicken ES cells, as compared to cells at EGK.VIII, EGK.X and HH4 stages, revealed a close relationship between chicken ES cells and blastodermal cells at the EGK.X stage, from which the ES cells were derived (Fig. 3b, based on PC1). However, notable differences were observed between ES cells and EGK.X cells, resulting in their separation on PC2. As an extra test, we identified signature genes from EGK.I to HH4 stages and performed heatmap analysis, which demonstrated that ES cells and EGK.X cells clustered together and were distinct from cells at other developmental stages (Extended Data Fig. 4d).
Subsequently, we conducted a comprehensive transcriptomic analysis of chicken ES cells, their in ovo counterpart EGK.X cells and HH4 cells at the gastrulation stage. At the whole-transcriptome level, chicken ES cells exhibited a higher linear correlation with EGK.X cells (R = 0.8), compared to HH4 cells (R = 0.73) (Extended Data Fig. 4e). Gene set enrichment analysis further confirmed that the expression of EGK.X signature genes, rather than HH4 signature genes, was significantly upregulated in chicken ES cells (Extended Data Fig. 4f).
By comparing gene expression in chicken ES cells with cells from EGK.X and HH4 embryos, we categorized all genes into three groups: A, B and C (Fig. 3c and Extended Data Fig. 4g). Group A contained 1,386 genes, with 358 upregulated and 1,028 downregulated in both chicken ES cells and EGK.X stage embryos relative to HH4 stage embryos. Within this group, core pluripotency genes, such as Nanog, Pou5f3 and Klf4 were highly expressed in chicken ES cells and EGK.X embryos (Fig. 3d). Gene ontology term enrichment analysis of the downregulated genes in group A revealed numerous differentiation and development-related gene ontology terms, suggesting that the OT/3i/chLIF condition effectively suppressed ES cell differentiation (Fig. 3e,f).
Group B contained 1,046 genes that showed no statistical difference between EGK.X and HH4 stage embryos but were significantly upregulated (384 genes) or downregulated (662 genes) in chicken ES cells. Gene ontology term analysis revealed that the upregulated genes were primarily involved in metabolic processes, suggesting that the differential expression between chicken ES cells and cells from EGK.X and HH4 stage embryos could be partially attributed to the environmental differences between in vitro and in ovo conditions (Extended Data Fig. 4g,h). Group C included the remaining 21,944 genes. Of these, 20,913 showed no statistically significant differences among chicken ES cells, EGK.X and HH4 embryos. The other 1,031 genes were differentially expressed between EGK.X and HH4 stage embryos but showed no significant differences between chicken ES cells and HH4 embryos. However, a gene ontology term analysis of these 1,031 genes did not reveal any statistically significant gene clusters (Padjust < 0.05).
In our bulk RNA-seq experiment, EGK.X embryos were collected in their entirety, comprising not only epiblast cells but also hypoblast cells and cells from the extra-embryonic region (area opaca). To further investigate the identity of chicken ES cells, we analyzed the expression of marker genes specific to the hypoblast and area opaca regions in both EGK.X embryos and cultured chicken ES cells39,40. This comparative analysis revealed that chicken ES cells exhibit low expression of markers associated with those two lineages, suggesting that the cultured ES cells closely resemble epiblast cells at the EGK.X stage (Fig. 3g).
In mice, at least three pluripotent states (naive, formative and primed) have been proposed18. To assess the pluripotent state of chicken ES cells, we conducted transcriptome correlation analysis using overlapping gene orthologs between two species and observed that chicken ES cells exhibited a closer resemblance to mouse formative pluripotent stem cells than to mouse naive or primed pluripotent stem cells (Fig. 3h).
In summary, our findings demonstrate that the OT/3i/chLIF condition effectively captures and maintains chicken ES cells in a pluripotent state resembling that of in ovo chicken EGK.X-stage epiblast cells.
Chicken ES cells are amenable to precise genome editing
To assess the genome-editing capability of chicken ES cells, we used a GFP-to-blue fluorescent protein (BFP) conversion assay to quantify CRISPR–Cas9-mediated genome editing via nonhomologous end joining and homology-directed repair41,42. A GFP-positive chicken ES cell line was derived from a transgenic chicken ubiquitously expressing GFP (Roslin Green)43. These cells were electroporated with Cas9 protein, a GFP single-guide RNA, and a single-stranded oligodeoxynucleotide (ssODN) donor to induce GFP-to-BFP knock-in conversion (Extended Data Fig. 5a). Flow cytometry analysis revealed a GFP-to-BFP conversion efficiency (BFP⁺/GFP−) of 9.3%, with an extra 3.7% of cells displaying single-allele conversion (BFP+/GFP+) (Extended Data Fig. 5b). Clonal populations were subsequently established via fluorescence-activated cell sorting and low-density plating, followed by expansion (Extended Data Fig. 5c). Genotypic analysis confirmed precise genome editing consistent with the observed fluorescence profiles (Extended Data Fig. 5d). These findings demonstrate that chicken ES cells are amenable to efficient and precise genome editing, supporting their use as a robust platform for both basic research and biotechnological applications.
Avian ES cells express core pluripotency factors
To characterize the pluripotency identity of avian ES cells beyond chicken, we examined ES cell lines derived from quail, goose and ostrich. Similar to chicken ES cells, these ES cell lines retained alkaline phosphatase activity, although the level and pattern of expression varied among colonies and species (Extended Data Figs. 2g,h, 3f,h and 6a). Quail ES cells demonstrated the capacity for in vitro differentiation into all three germ layers, as evidenced by the expression of TUJ1 (ectoderm), MHC (mesoderm) and GATA4 (endoderm) (Extended Data Fig. 6b). To further explore transcriptional identity, we performed bulk RNA-seq on ES cell lines derived from quail, goose and ostrich, and compared them to chicken ES cells. All four avian ES cell lines expressed high levels of key pluripotency-associated transcription factors, including Nanog or Nanog-like, Lin28a/b and Pou5f3, while other Pou family members were not detected (Extended Data Fig. 6c). We also examined the expression of SOX and KLF family genes. Unlike mammalian ES cells, avian ES cells did not express Sox2; instead, Sox3 was consistently expressed in ES cells derived from all four avian species. In addition, Sox4 and Sox11 were coexpressed in quail and goose ES cells. Regarding KLF family genes, avian ES cells predominantly expressed Klf3, Klf5 and Klf6, in contrast to the high Klf4 expression observed in mammalian ES cells. Notably, the expression levels of Klf3/5/6 varied among the different avian ES cell lines.
Avian ES cells show species-specific responses to LIF signaling
While chicken, quail and goose ES cells exhibited improved self-renewal in the presence of exogenous LIF, ostrich ES cells responded differently: LIF exposure induced differentiation, potentially toward trophectoderm lineages (Extended Data Fig. 3e–h). Transcriptomic profiling revealed that ostrich ES cells uniquely expressed elevated levels of LIF receptor (LIFR) and Gp130 (IL6ST) compared to other avian ES cell lines (Extended Data Fig. 6d). This observation aligns with previous mechanistic studies in mouse ES cells, where STAT3 signaling functions as a dose-sensitive ‘rheostat’ rather than a binary switch in regulating cell fate44. Together, these findings suggest that while ES cells derived from diverse avian species share core pluripotent features with chicken ES cells, they also display species-specific regulatory response, particularly in their sensitivity to the LIF/STAT3 signaling pathway.
Chicken ES cells derived in OT/3i/chLIF are chimera-competent
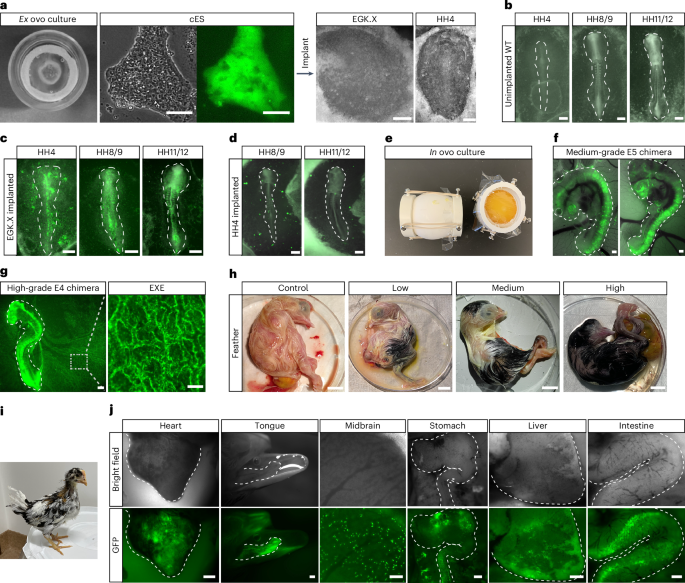
The ability to contribute to chimera formation is a defining characteristic of authentic ES cells. To investigate whether OT/3i/chLIF chicken ES cells possess this capacity, we implanted them onto ex ovo incubated chicken embryos (Fig. 4a). GFP-positive chicken ES cells efficiently integrated into the developing chicken EGK.X-stage embryos, leading to the formation of chimeric embryos (Fig. 4b,c). In contrast, chicken ES cells implanted into HH4 stage embryos failed to integrate (Fig. 4d), highlighting the importance of matching developmental stages between donor cells and recipient embryos45,46.
a, GFP-labeled chicken ES cells were implanted onto ex ovo cultured EGK.X or HH4 chicken embryos. Scale bars, 100 μm (cES cells), and 400 μm (EGK.X and HH4 embryos). b, Representative images showing HH4-HH11/12 stage embryos developed from ex ovo cultured chicken EGK.X embryos without the implantation of chicken ES cells. Scale bars, 400 μm. c, Representative images showing HH4-HH11/12 stage embryos developed from ex ovo cultured chicken EGK.X embryos implanted with GFP-positive chicken ES cells. Scale bars, 400 μm. d, Representative images showing HH8/9 and HH11/12 stage embryos developed from ex ovo cultured chicken HH4 embryos implanted with GFP-positive chicken ES cells. Scale bars, 400 μm. e, Image of the surrogate open-shell system for in ovo chimeric embryo development. f,g, Chimeras generated by injecting GFP-positive chicken ES cells into EGK.X-stage chicken embryos: medium-grade E5 chimera (f) and high-grade E4 chimera (g). Scale bars, 400 μm. EXE, extra-embryonic blood vessels (scale bar, 100 μm). h, Images showing control, low-grade, medium-grade and high-grade E20 chimeras generated by injecting RIR chicken ES cells into EGK.X White Leghorn chicken embryos. Scale bar, 10 mm. i, A chimeric chicken with black feathers from donor ES cells derived from RIR at 31 days old posthatch. Recipient embryo, White Leghorn. j, Images showing GFP-positive cells in the indicated organs of chimeras generated by injecting GFP-positive chicken ES cells into EGK.X chicken embryos. Heart (E13, scale bar, 1 mm), tongue (E20, scale bar, 1 mm), midbrain (E8, scale bar, 100 μm), stomach (E7, scale bar, 400 μm), liver (E21, scale bar, 400 μm) and intestine (E19, scale bar, 400 μm). Chimeras generated are from 1 representative experiment of more than 5 experiments performed in a–d, for ex ovo, and more than 100 experiments performed in e–j, for in ovo.
While the ex ovo culture system provided a straightforward means to assess the chimera formation potential of chicken ES cells, the in ovo system was necessary for long-term monitoring of donor cell development. We used the surrogate open-shell system to observe chimeric embryo development until hatching47 (Fig. 4e and Extended Data Fig. 7a). GFP-positive chicken ES cells were injected into the subgerminal cavity of EGK.X-stage chicken embryos. Embryonic development and the distribution of GFP-positive cells were monitored through a window sealed with plastic film (Extended Data Fig. 7b). Chicken ES cells contributed to multiple lineages within the chicken embryos in ovo (Extended Data Fig. 7c and Supplementary Video 2). However, the overall level of in ovo chimerism was relatively low compared to ex ovo chimeras.
Previous studies demonstrated that sublethal irradiation of recipient chicken embryos can enhance the contribution of injected blastodermal cells48. Applying this method, irradiation of recipient embryos at 500–550 cGy (rad) before chicken ES cell injection increased the extent of chimerism without substantially compromising embryo viability (Fig. 4f,g and Extended Data Fig. 7d). Long-term cultured chicken ES cells, which included 60 passages on feeders and 15 passages under feeder-free conditions, retained their capacity for chimera formation (Extended Data Fig. 7e). Immunostaining for lineage markers in chimeric embryos demonstrated donor cell contribution to neural tube formation, indicated by SOX2 expression, and to the mesoderm, marked by Brachyury expression (Extended Data Fig. 7f). Notably, chicken ES cells gave rise to both somatic and extra-embryonic cells in the chimeric embryos (Fig. 4g and Supplementary Videos 3 and 4), a feature distinct from mouse ES cells.
To assess the sustainability of functional engraftment over time, we analyzed chimeric embryos at later developmental stages (embryonic day 8–21 and posthatching). Visible coat color chimeras were successfully obtained, consistent with the chimera rate determined by GFP coverage at E4 (Fig. 4h,i). Furthermore, we detected donor cell contribution to organs from multiple germ layers (Fig. 4j). A summary of the avian ES cell–embryo chimeras is provided in Supplementary Table 3, and the ratios of chimerism in chicken ES cell–chicken embryo chimeras generated via nonirradiation and irradiation methods are provided in Extended Data Fig. 7d. Collectively, the ex ovo and in ovo experiments demonstrated the efficient integration of chicken ES cells into embryonic development, contributing to both somatic and extra-embryonic cell lineages.
Chicken ES cells contribute to feather patterning in chimeras
To further monitor the development of engrafted chicken ES cells over the long-term, embryos with feather color chimerism were hatched and raised for observation (Fig. 4i and Extended Data Fig. 8a). In adult feathers, the feather follicle undergoes cyclic renewal, and the pigment patterns depend on the follicle melanocyte stem cells49. The White Leghorn chicken strain has a mutation that affects melanocytes’ ability to process melanosome properly, resulting in an apigmented phenotype50. We investigated whether chicken ES cells derived from RIR could contribute to the functional melanocyte lineage and exhibit pigmentations in the chimera. Contour feather follicles were collected from adult chimeric chickens and compared to feathers from adult White Leghorn and RIR chickens (Extended Data Fig. 8b–d). Paraffin sections were stained with hematoxylin and eosin, revealing normal feather follicle development in chimeric chickens (Extended Data Fig. 8e–g). Bright views of feather follicles displayed pigmented chimeric feathers compared to apigmented White Leghorn feathers (Extended Data Fig. 8h–j). Staining for microphthalmia-associated transcription factor (MITF), a marker for melanocyte progenitor cells51, showed MITF expression in RIR and chimeric chicken feathers, while White Leghorn chicken feathers were MITF-negative (Extended Data Fig. 8k–m). In addition, chimeric chicken feathers were GFP-positive (Extended Data Fig. 8n). All together, these findings indicate that chicken ES cells derived from RIR contributed to the generation of melanocyte progenitor cells inside the recipient White Leghorn feather follicles, resulting in feather pigmentation in chimeric chickens.
Avian ES cells contribute to intra- and interspecies chimeras
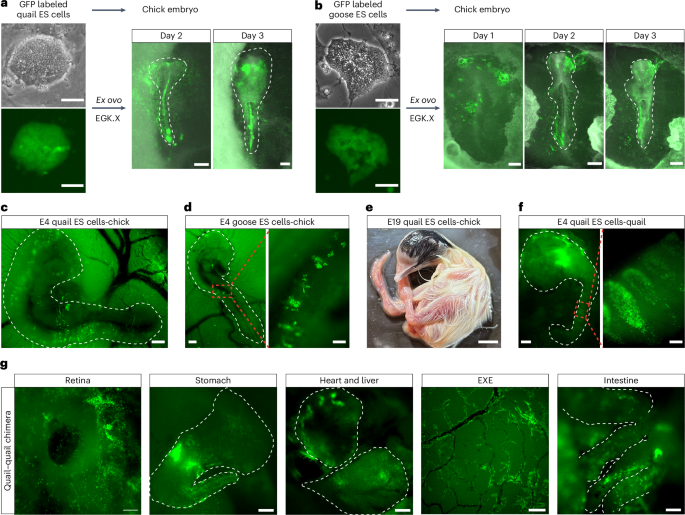
To evaluate the developmental potential of ES cells derived from other avian species, we generated quail and goose ES cells that constitutively expressed GFP (Fig. 5a,b). Given that quail-chick chimeras can be created by injecting quail blastodermal cells into chicken embryos52, we hypothesized that this interspecies chimera system could be used to assess the chimera competency of avian ES cells. GFP-positive quail and goose ES cells were implanted onto ex ovo incubated EGK.X-stage chicken embryos, resulting in the successful integration of donor cells into the recipient chicken embryos (Fig. 5a,b).
a, GFP-labeled quail ES cells (left panel, scale bars, 50 μm) were implanted onto EGK.X chicken embryos, and the contribution of quail ES cell-derived cells in the chicken embryos was traced ex ovo for 3 days. Embryo scale bars, 220 μm. b, GFP-labeled goose ES cells (left panel, scale bars, 50 μm) were implanted onto EGK.X chicken embryos, and the contribution of goose ES cell-derived cells in the chicken embryos was traced ex ovo for 3 days. Embryo scale bars, 220 μm. c,d, Representative images showing ES cell–chicken embryo chimeras generated by in ovo injection of GFP-labeled quail (c, scale bar, 400 μm) or goose (d, scale bars, 400 μm, left; 100 μm, right) ES cells into the subgerminal cavity of EGK.X chicken embryos. e, An E19 quail ES cell–chicken embryo chimera showing the contribution of quail ES cells (black feathers) in the chimera. Scale bar, 10 mm. f, Images showing a quail ES cell–quail embryo chimera generated by injecting GFP-labeled quail ES cells into the subgerminal cavity of an EGK.X quail embryo. Scale bars, 400 μm (left), 100 μm (right). g, Images showing GFP-labeled quail ES cell-derived cells inside the indicated quail organs. E6 retina, scale bar, 100 μm; E8 stomach, scale bar, 400 μm; E8 heart (top) and liver (bottom), scale bar, 400 μm; E8 extra-embryonic blood vessels (EXE), scale bar, 400 μm; E8 intestine, scale bar, 100 μm. Chimeras generated are from 1 representative experiment of more than 3 experiments performed in a–b, for ex ovo, and more than 10 experiments performed in c–g, for in ovo.
To further confirm the chimera formation capacity of avian ES cells, we injected GFP-positive quail and goose ES cells into chicken embryos in ovo. Both quail and goose ES cells contributed to chimera formation within developing chicken embryos (Fig. 5c,d). Notably, donor ES cells from quail and goose participated in the somitogenesis process in recipient chicken embryos. The stable integration of quail ES cells within chicken embryos was evidenced by coat color chimerism, with black feathers from donor quail cells appearing on an apigmented White Leghorn chicken embryo (Fig. 5e). In addition, GFP-positive donor cells from quail and goose were found in the blood circulation, indicating their contribution to the blood lineage in the recipient chicken embryos (Supplementary Videos 5 and 6).
Finally, substantial integration was observed when quail ES cells were injected into the subgerminal cavity of quail embryos in ovo, with donor cells contributing to organs derived from multiple germ layers (Fig. 5f,g and Supplementary Video 7). These findings demonstrate that quail and goose ES cells can efficiently contribute to chimera formation.
Avian ES cells can differentiate into germ cells
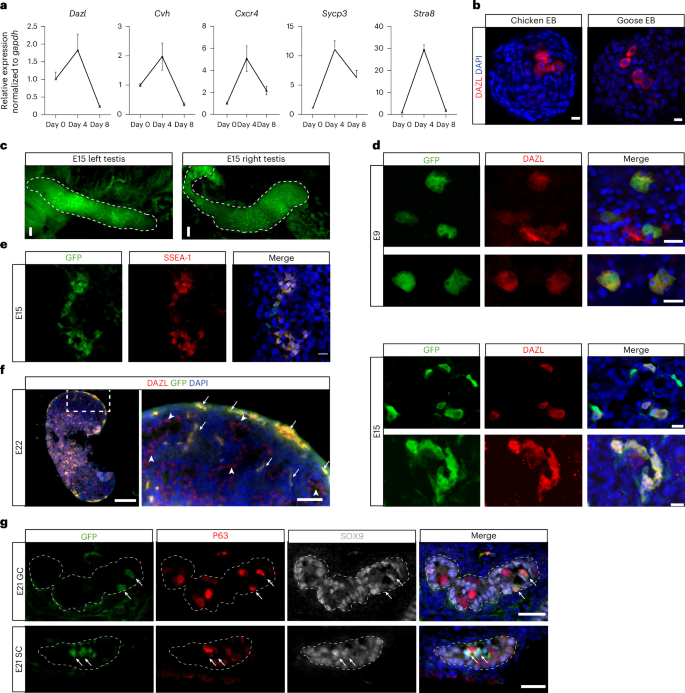
Next, we evaluated the germ cell differentiation potential of chicken ES cells. We generated EBs from chicken ES cells and examined the expression of germ cell markers, including Dazl, Cvh, Cxcr4, Sycp3 and Stra8 (ref. 53). The relative expression levels of these markers significantly increased in day-4 EBs compared to undifferentiated chicken ES cells, but subsequently decreased in day-8 EBs, likely due to the expansion of somatic cells within the EBs (Fig. 6a). This peak in germ cell marker expression identified day 4 as an optimal time point to further investigate germ cell formation in EBs.
a, RT–qPCR analysis of the expression of germ cell markers in chicken ES cells (day 0) and during EB differentiation. Data represent mean ± s.d. of three biological replicates. b, Immunofluorescence staining of DAZL in day-3 EBs formed from chicken or goose ES cells. n = 3 independent experiments. Scale bars, 10 μm. c, GFP fluorescence images showing the testes dissected from an E15 high-grade chimera generated by injecting GFP-labeled chicken ES cells into the subgerminal cavity of an EGK.X chicken embryo. Scale bars, 50 μm. d, Images showing GFP and DAZL double staining of tissue sections from E9 and E15 testes dissected from chimeras generated by injecting GFP-labeled chicken ES cells into the subgerminal cavity of EGK.X chicken embryos. Scale bars, 10 μm. e, Images showing GFP and SSEA-1 double staining of tissue sections from an E15 gonad dissected from a chimera generated by injecting GFP-labeled chicken ES cells into the subgerminal cavity of an EGK.X chicken embryo. Scale bars, 10 μm. f, Immunostaining of E22 testis sections from a chimera generated by injecting GFP-labeled chicken ES cells into the subgerminal cavity of an EGK.X-stage chicken embryo. The right panel shows a zoom-in view of the boxed area in the left panel. Arrows indicate GFP and DAZL double-positive cells; arrowheads indicate GFP-negative/DAZL-positive cells. Scale bars, left, 200 µm, right, 50 µm. g, Images showing GFP/P63/SOX9 triple staining of tissue sections from E21 testes dissected from chimeras generated by injecting GFP-labeled chicken ES cells into the subgerminal cavity of EGK.X chicken embryos. Arrows indicate GFP+/P63+/SOX9− germ cells (GC) in the top row and GFP+/P63−/SOX9+ Sertoli cells (SC) in the bottom row. Scale bars, 20 μm. Chimeras shown in c–g are from one representative experiment out of five performed.
To further investigate germ cell differentiation, we performed immunofluorescence staining for the PGC marker DAZL in both undifferentiated chicken ES cells and differentiating EBs. DAZL expression was not detected in chicken ES cells, but a subset of cells (1–3%) in day-3–4 EBs stained positive for DAZL, including those derived from goose ES cells (Fig. 6b, Extended Data Fig. 9a and Supplementary Video 8). Immunostaining of cultured goose ES cells showed a consistent, albeit very low percentage (0.01–0.14%, n = 7) of DAZL-positive cells without the need of EB formation. To determine whether these DAZL-positive cells originated from cultured ES cells or residual PGCs present in the initial culture, goose ES cells were seeded at low density to allow clonal expansion. Individual colonies were picked and expanded to establish clonal lines (n = 4). DAZL-positive cells were still detected in all clonal lines, confirming that cultured goose ES cells possess the capacity to generate DAZL-positive cells in vitro (Extended Data Fig. 9b). To further characterize ES cell-derived PGC-like cells, we designed a probe targeting Cvh for in situ hybridization chain reaction and validated its specificity in chicken HH8 embryo (Extended Data Fig. 9c). Using this probe, we also found Cvh-positive cells inside the chicken EBs (Extended Data Fig. 9d).
To examine germline contribution in ovo, we injected male GFP-positive chicken ES cells into EGK.X-stage chicken embryos pretreated with 500–550-cGy irradiation. Sublethal irradiation has been shown to increase the germline transmission rate of donor PGCs in chickens by reducing the number of endogenous PGCs54. All subsequent analysis of donor ES cell-derived germ cells were performed exclusively using male-to-male donor–recipient pairings. GFP-positive donor cells were present in the gonads of chicken embryos injected with chicken ES cells, particularly in high-grade chimeras (Fig. 6c).
We analyzed the presence of ES cell-derived germ cells in chimeric embryos at different developmental stages. In E9 chimeric embryos, DAZL-double-positive and GFP-double-positive cells were present in gonads as small clusters consisting of 1–3 cells (Fig. 6d). In E15 and later chimeric embryos, larger clusters of GFP-positive cells that were also positive for DAZL or SSEA-1, another germ cell marker55, were found in the gonads (Fig. 6d–f and Extended Data Fig. 9e). In E21 embryos, some PGCs were enclosed within the seminiferous tubules as gonads continued to develop. GFP-positive PGCs, identified by staining with P63, a germ cell marker56, could be detected within the tubules alongside endogenous PGCs. Furthermore, some seminiferous tubules contained GFP cells that differentiated into Sertoli cells, as indicated by staining with SOX9 (Fig. 6g).
Multiple chicken ES cell lines were confirmed to contribute to the germline lineage within chicken embryos (Supplementary Table 4). Notably, chicken ES cell lines that failed to differentiate into DAZL-positive cells in vitro were able to give rise to germ cells after transplantation into chicken embryos, suggesting a more favorable germline-inductive environment in ovo compared to the spontaneous differentiation environment in vitro. Qualitative analysis indicated that high-grade chimeras exhibited a substantial proportion of donor-derived PGCs (Fig. 6d,e and Supplementary Table 4). In contrast, donor-derived PGCs were present at low frequencies in mid-grade chimeras and were not observed in low-grade chimeras (Supplementary Table 4). In conclusion, by tracking the germline development of avian ES cells, we demonstrated their competence to adopt a germ cell fate both in vitro and in ovo.