Method for ultrastructural imaging of the mouse brain
Extended Data Fig. 1 shows an overview of our brain tissue pan-ExM-t protocol. In brief, mice are transcardially perfused with fixative containing both formaldehyde (FA) and acrylamide (AAm) and their brains are extracted surgically and incubated in the same fixative overnight at 4 °C. The brains are then sectioned at 50–100-µm thickness using a vibratome and stored in PBS until future use. Each tissue section to be expanded is embedded in a dense poly(acrylamide/sodium acrylate) copolymer that is crosslinked with N,N′-(1,2-dihydroxyethylene)bis-acrylamide (DHEBA), an AAm crosslinker with a cleavable amidomethylol bond. After polymerization, the now tissue–hydrogel hybrid is denatured with sodium dodecyl sulfate (SDS) in heated buffer (pH 6.8) for 4 hours and expanded roughly fivefold in deionized water. Next, a specific region of interest (~8 × 8 mm2) is cut and re-embedded first in a neutral polyacrylamide hydrogel crosslinked with DHEBA and then in a poly(acrylamide/sodium acrylate) copolymer crosslinked with N,N′-methylenebis(acrylamide) (BIS), a nonhydrolyzable AAm crosslinker. As we previously demonstrated18, no secondary fixation of proteins before the re-embedding is required. The sample is then incubated in 200 mM sodium hydroxide to cleave DHEBA and thereby remove crosslinks of the first and second hydrogel polymer and linearize them. After neutralization with multiple PBS washing steps, the sample is labeled with antibodies, pan-stained with fluorescent dyes to show protein-dense areas, washed with detergents, and expanded to its final size of ~16–24-fold in ultrapure water. The expanded (and, as a side-effect, optically cleared) sample is finally imaged on a standard confocal microscope and can be stored at 4 °C for months.
pan-ExM reveals synapse ultrastructure in dissociated neurons
Before experimenting with expanding mouse brain tissue sections, we tested pan-ExM in dissociated hippocampal rat and mice neurons using our published protocol with modifications in sample fixation (Methods). A visual comparison of N-hydroxysuccinimide (NHS) ester bulk amine pan staining of neurons that are nonexpanded (Fig. 1a) or expanded with pan-ExM (Fig. 1b–k, Supplementary Fig. 1 and Supplementary Videos 1 and 2), illustrates this approach: nonexpanded synapses show essentially uniform staining, revealing little information, whereas ~16-fold expanded synapses can be spatially resolved by their protein density patterns. Analogous to phosphotungstic acid (PTA-)staining of neurons in EM23,24 (Fig. 1l), pan-ExM now resolves nanoscale features such as dense projections (DPs) of the presynaptic bouton (Fig. 1m, lime arrow) and the PSD (Fig. 1m, salmon arrow), allowing for the identification of synapses by their morphological characteristics. We see spines that are stubby (Fig. 1d,f,i), mushroom-shaped (Fig. 1h,j) and thin (Fig. 1g,k). We also observe hexagonal protein-dense patterns formed by presynaptic DPs (Fig. 1e) a discovery made in the 1960s with EM25,26. To determine the achieved linear expansion factors, we imaged SYTOX Green-stained neuron nuclei in nonexpanded samples and samples expanded using our standard protocol and compared the average nuclear cross-sectional area (Supplementary Methods). On average, we obtained an expansion factor of 15.7 ± 0.3 (mean ± s.d.; N = 4 experiments; n = 6–13 nuclei per experiment). Dividing the measured distances between DPs and the PSD (center to center) by the expansion factor determined from nuclei measurements, we obtained a value of 82 ± 26 nm (mean ± s.d.; N = 4 experiments; n = 44 synaptic profiles; Fig. 1n), which is consistent with the range of presynaptic density and PSD distance measurements determined previously by super-resolution microscopy and electron tomography (~70–115 nm)27,28,29,30,31. Similarly, dividing the distance between neighboring DPs by the nuclear expansion factor, we obtained a value of 67 ± 15 nm (mean ± s.d.; N = 6 experiments; n = 78 DP profiles; Fig. 1o), consistent with earlier EM data28,32 (~60–80 nm). In all subsequent experiments, we used the DP–PSD distance as a metric for linear expansion factor calculation (Supplementary Methods).
a, NHS ester pan-stained dendrite in a nonexpanded sample showing dendritic spines. b, Axial view model of a synapse showing DPs and synaptic vesicles (SVs) in the presynaptic bouton, and the PSD, and the active zone (AZ) in the postsynaptic dendritic spine. c, Top view of a synapse showing hexagonal DPs in the presynaptic bouton and SV attachment sites. d,f–k, pan-ExM processed and NHS ester pan-stained spines including mushroom (h,j), stubby (d,f,i) and thin (g,k) shapes. e, NHS ester pan-stained synapse showing hexagonally arranged DPs. l, Transmission EM (TEM) image of a PTA-stained synapse showing prominent DPs (lime arrows) and a PSD (salmon arrow). m, pan-ExM processed and NHS ester pan-stained synapse for comparison, showing similar hallmark ultrastructural features. n, DP–PSD distances (n = 44 measurements from 4 independent experiments). o, DP–DP distances (n = 78 measurements from 6 independent experiments). p, Comparison of Bassoon–PSD and DP–PSD distances (n = 50 measurements from 3 independent samples, Welch’s two-sided t-test, P = 0.035). q, Comparison of DP–Homer1 and DP–PSD distances (n = 85 measurements from 4 independent samples, Welch’s two-sided t-test, P = 0.006). r, Comparison of PSD-95–DP and DP–PSD distances (n = 25 measurements from 2 independent samples, Welch’s two-sided t-test, P = 0.049). *P < 0.05; **P < 0.01. s, Relative spatial distributions of Bassoon, PSD-95 and Homer1 along the trans-synaptic axis. Statistical significance was assessed by Welch’s two-sided t-tests, and medians and interquartile ranges are shown with whiskers drawn down to the minimum and maximum values. t,w,z, Axial (t,w) and top (z) views of synapses pan-stained with NHS ester. u,x,aa, Bassoon immunolabeling of the same areas. v,y,bb, Respective overlays. cc–kk,ll–qq,rr–ww,xx–ccc, Same as t–bb in samples labeled for Homer1, labeled for PSD-95, double-labeled for Homer1 and Bassoon, and labeled for Synaptophysin (SYN), respectively. The inset in ccc shows SYN puncta, representing synaptic vesicles, intercalated between neighboring DPs. ddd, NHS ester image of a mitochondrion in a hippocampal rat neuron. mtDNA, mitochondrial DNA. eee, SYTOX Green (SYX) staining of the same area. fff, Overlay. ggg, NHS ester image of a nuclear pore complex (NPC). hhh, Overlay of ggg with a SYTOX Green image of the same area. iii, NHS ester image of basal bodies in a mouse neuron. The inset shows the familiar centriolar cartwheel structure. jjj, NHS ester image of a cilium in a mouse neuron. Lime and salmon arrows point to the basal body and ciliary tip, respectively. Medians and interquartile ranges are shown with whiskers drawn down to the minimum and maximum values. Panels showing pan staining or TEM (l) are displayed with a white-to-black color table; panels showing immunolabeling or SYTOX Green staining are displayed with a black-to-white color table. Gamma corrections: d,e, γ = 0.8; h,j,k,m,cc,ll,oo,rr,uu,xx,aaa,iii,jjj, γ = 0.7; i, γ = 0.6. jjj is a z-projection (intensity average) of five images. All scale bars are corrected for the expansion factor. Scale bars: 800 nm (a), 200 nm (d–m,t–ccc, jjj), 300 nm (ddd–fff), 50 nm (ggg,hhh), 100 nm (iii). Expansion factor: 16 ± 2.
pan-ExM in dissociated neurons is compatible with immunofluorescence labeling as well as other established chemical stainings, enabling correlative studies that combine specific and contextual pan-staining approaches. Focusing on the synapse, Fig. 1t–ccc and Supplementary Figs. 2–4 show the distributions of synaptic proteins Bassoon, Homer1, PSD-95 and Synaptophysin in the context of synaptic ultrastructure. We observe compartmentalization of active zone protein Bassoon into distinct puncta as DPs (Fig. 1t–bb) with synaptic vesicle protein Synaptophysin intercalating in between neighboring DPs (Fig. 1xx–ccc), supporting the model that DPs represent distinct sites for synaptic vesicle docking and fusion at the active zone. We also observe nanoclustering of PSD proteins Homer1 and PSD-95 along an otherwise macular and dense PSD, with Homer1 slightly offsetting the PSD further into the spine (Fig. 1cc–kk,q) and PSD-95 concentrating directly over the PSD (Fig. 1ll–qq,r), consistent with previous work31,33. The distance between Bassoon and the PSD is 104 ± 24 nm (mean ± s.d.; N = 3 independent samples; n = 50 synaptic profiles; Fig. 1p,s, compared with 90–120 nm reported previously31); the distance between Homer1 and the DP is 83 ± 16 nm (mean ± s.d.; N = 4 independent samples; n = 85 synaptic profiles; Fig. 1q,s) and the distance between PSD-95 and the DP is 68 ± 11 nm (mean ± s.d.; N = 2 independent samples; n = 25 synaptic profiles; Fig. 1r,s). The measured distance between Bassoon and Homer1 of ~90–120 nm is consistent with previous reports using super-resolution imaging (compare with 70–127 nm, ref. 29 or 90–155 nm, ref. 31). Double immunostainings, for example of Bassoon and Homer1 (Fig. 1rr–ww, Supplementary Fig. 5 and Supplementary Video 3), are also compatible with pan-ExM, offering detailed structural information that is inaccessible by conventional confocal microscopy of unexpanded or only roughly fivefold expanded samples (Supplementary Fig. 6).
Similarly, pan-ExM can clearly resolve subcellular structures too small to resolve without expansion, including mitochondrial nucleoids and cristae (Fig. 1ddd–fff), the hollow, circular structure of nuclear pore complexes (Fig. 1ggg–hhh) and the cartwheel structure of basal bodies, their distal appendages and the ciliary tip of cilia (Fig. 1iii–jjj). Furthermore, by combining NHS ester pan staining with metabolic incorporation of palmitic acid azide, it becomes possible to examine the contact sites of membranous organelles, such as between tubules of the endoplasmic reticulum and mitochondria (Supplementary Fig. 8), and other subcellular neuronal features (Supplementary Figs. 9 and 10).
pan-ExM-t reveals tissue ultrastructural features
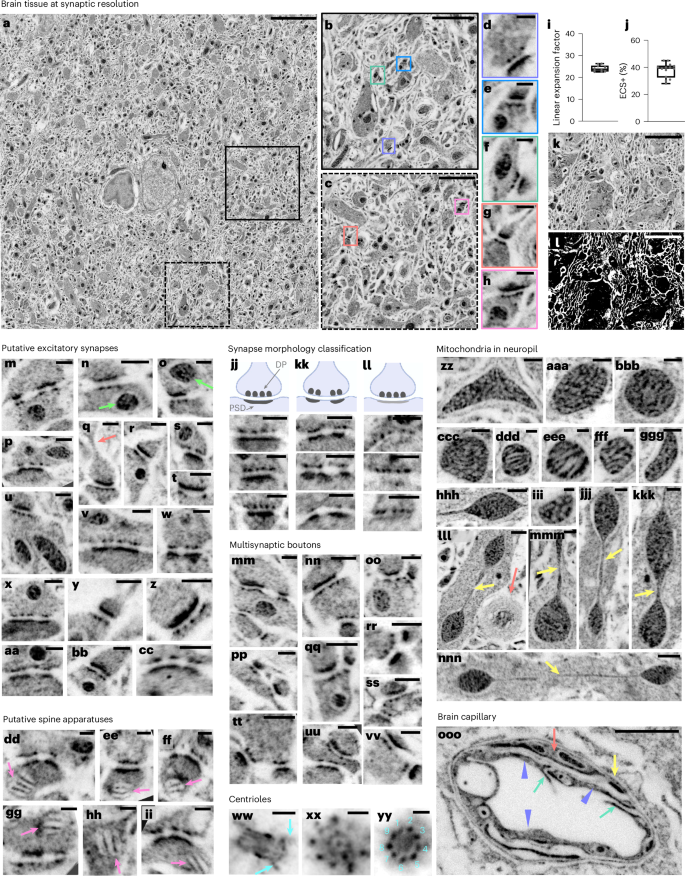
Having established pan-ExM in dissociated neuron cultures, we adapted our technique to 70-µm-thick mouse brain tissue sections. Because of stark differences in thickness, lipid content and presence of a highly connected extracellular matrix in tissue that is absent in dissociated neurons, brain fixation, sample denaturation and antibody labeling parameters had to be optimized. After comparing many different fixation strategies (Supplementary Methods and Supplementary Figs. 11–14), we found that using 4% formaldehyde + 20% AAm in both the transcardial perfusion and overnight postfixation solutions achieves good neuropil preservation, very little artifactual tissue perforations (Fig. 2a–h), an acceptable extracellular space and lipid membrane (ECS+) fraction of ~38% (Supplementary Methods and Fig. 2j–l) and an expansion factor of 24.1 ± 1.4-fold (mean ± s.d.; N = 10 field of views (FOVs) from 3 independent experiments; n = 254 synaptic profiles, Fig. 2i). We therefore decided to use this fixation strategy for all subsequent experiments.
a, NHS ester pan-stained tissue section of the mouse cortex. b,c, Magnified areas in the black and dotted black boxes in a, respectively. d–h, Magnified areas identified by the correspondingly colored boxes in b,c showing putative excitatory synapses. i, Linear expansion factor (n = 254 measurements from 3 independent experiments, corresponds to data shown in a–h). j, ECS + lipid membrane (ECS+) fraction (n = 14 measurements from 3 independent experiments). k, Image of neuropil in the hippocampus. l, Same area as k where white pixels represent the ECS+ fraction. m–cc, Putative excitatory synapses defined by a prominent PSD. Lime arrows in n and o point to mitochondria in the presynaptic bouton and in the postsynaptic compartment, respectively. The salmon arrow in q points to a spine neck. dd–ii, Putative spine apparatuses (pink arrows) in the postsynaptic compartment defined by a characteristic lamellar arrangement. jj–ll, Classification of synapses based on the patterns and intensity of the PSD. jj, Type I (asymmetric) synapses where the PSD is prominent and macular. kk, Type I (asymmetric) synapses where the PSD is prominent and perforated. ll, Type II (symmetric) synapses, where the PSD, unlike DPs, is thin or barely visible. mm–vv, NHS ester pan-stained multisynaptic boutons and their postsynaptic partners. ww, Lateral view of a centriole showing distal and proximal ends as well as distal appendages (turquoise arrows). xx,yy, Top views of centrioles showing the cartwheel structure (xx) and the ninefold symmetry of microtubule triplets (yy). zz–nnn, Mitochondria with vesicular cristae (zz–ccc,ggg,iii), lamellar cristae (ddd–fff) and teardrop-shaped with tubular extensions (yellow arrows) (jjj–nnn). Salmon arrow in lll points to putative myelinated sheaths. ooo, Brain capillary showing endothelial cells (lavender arrow heads), putative tight junctions (TJs; teal arrows) that link neighboring endothelial cells, putative pericyte branch (salmon arrow) and the basement membrane (yellow arrow). Medians and interquartile ranges are shown with whiskers drawn down to the minimum and maximum values. Panels showing pan staining are displayed with a white-to-black color table. Gamma corrections: a,ww–yy,ooo, γ = 0.7; m–vv,zz–nnn, γ = 0.8. All scale bars are corrected for the expansion factor. Scale bars: 5 µm (a), 1 µm (b,c,ooo), 200 nm (d–h,m–cc,jj–vv,xx,ww,zz–ccc,ggg,jjj,kkk), 3 µm (k,l), 100 nm (dd–ii,xx,yy,ddd–fff,iii,mmm; 400 nm (hhh,lll,nnn). Expansion factor for m–ooo: 21.3 ± 1.5.
Equipped with this pan-ExM-t protocol, we imaged a wide variety of tissue nanostructures across both hippocampal and cortical regions of the mouse brain. Figure 2a shows a tiled ~1 × 1-mm2 image (corresponding to ~42 × 42-µm2 after correction for the expansion factor) of NHS ester pan-stained cortical tissue at synaptic resolution. Hallmark synaptic features such as presynaptic densities and PSDs within a brain tissue section were revealed by standard confocal microscopy (Fig. 2d–h, Supplementary Figs. 15–17 and Supplementary Videos 4 and 5). For example, we could observe that putative excitatory synapses, defined by a prominent PSD (Fig. 2m–cc), often featured mitochondria in the vicinity of axonal boutons (Fig. 2n, arrow) and sometimes in the postsynaptic partner (Fig. 2o, arrow). We can discern densely stained stacked structures in some postsynaptic compartments, suggestive of spine apparatuses34 (Fig. 2dd–ii). Moreover, the obtained resolution allowed us to classify synapses based on variations in PSD patterns and intensities according to the previously established34,35,36,37 Gray classification of synapses: type I (asymmetric, excitatory) synapses with a thick PSD that can be either macular (Fig. 2jj) or perforated (Fig. 2kk); and type II (symmetric, inhibitory) synapses, where the PSD is thin or barely visible, despite prominent DPs in the presynaptic bouton (Fig. 2ll). Furthermore, pan-ExM-t images reveal the spine neck (Fig. 2q, arrow) and multisynaptic boutons (Fig. 2mm–vv).
Centrioles in the perikarya are distinguished by a bright NHS ester pan staining showing clear distal appendages (Fig. 2ww) and, in top views, the cartwheel structure (Fig. 2xx) and ninefold symmetry (Fig. 2zz). The ninefold symmetry is also visible in multiciliated ependymal epithelia lining the lateral ventricles of the mouse brain (Fig. 4l, Supplementary Video 6 and Supplementary Fig. 18). Moreover, we also observe that mitochondria morphologies vary strongly across neuropil (Fig. 2zz–nnn), featuring clearly resolvable lamellar as well as putative tubular cristae, and shapes ranging from vesicular to teardrops with tubular extensions (Fig. 2lll–nnn, arrows). These structures are likely not the result of fixation artifacts, as EM images of tissue preserved with similar protocols resolve mitochondrial ultrastructure well (Extended Data Fig. 4), but reflect native mitochondrial morphology that has been reported to correlate with disease and synaptic performance38,39. A closer look at brain capillaries (Fig. 2ooo and Supplementary Fig. 19) reveals clearly discernible endothelial cells, tight junctions, pericytes and the basement membrane37.
pan-ExM-t is compatible with antibody labeling in thick brain tissue sections
A particular strength of pan-ExM-t is its ability to localize specific proteins to subcompartments revealed in the contextual pan-stained channel. Our protocol must therefore allow for efficient antibody staining, while also maintaining good ultrastructural preservation. Because hydrogels with high monomer concentrations are known to impede antibody diffusion, we investigated whether lowering the monomer concentration in the different interpenetrating hydrogels would result in better signal-to-noise antibody stainings (Supplementary Methods). We found that a combination of 19% (w/v) sodium acrylate (SA) for the first expansion hydrogel followed by 9% (w/v) SA for the second expansion resulted in efficient labeling and low background following synaptic protein immunolabeling (Supplementary Fig. 20).
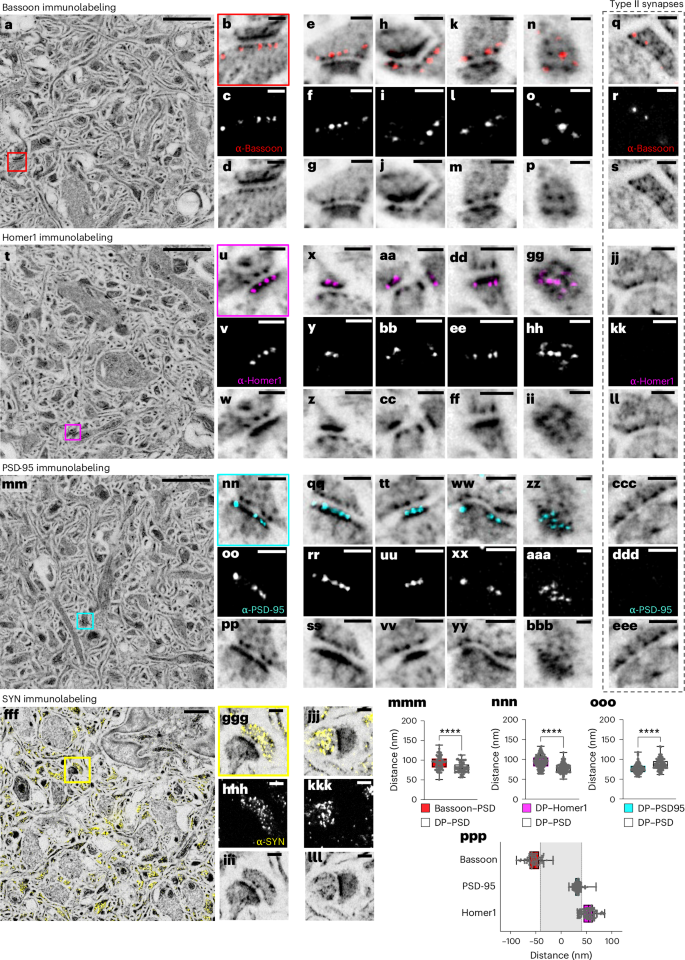
Using this robust pan-ExM-t protocol, we performed several immunostainings against commonly studied synaptic proteins Bassoon, Homer1, PSD-95 and Synaptophysin (Fig. 3 and Supplementary Video 6). The expansion-corrected distributions of Homer1, PSD-95 and Bassoon within the axial DP–PSD distances are all in agreement with published values29,31,33 as well as with values obtained from cultured neuron measurements in this work. For example, the distance between Bassoon and the PSD is 94 ± 17 nm (mean ± s.d.; N = 3 FOVs; n = 74 synaptic profiles; Fig. 3mmm, compared with 90–120 nm reported previously31); the distance between Homer1 and DP is 93 ± 16 nm (mean ± s.d.; N = 5 fields on views; n = 120 synaptic profiles; Fig. 3nnn) and the distance between PSD-95 and the DP is 76 ± 12 nm (mean ± s.d.; N = 3 FOVs; n = 113 synaptic profiles, Fig. 3ooo). Figure 3ppp shows a plot of the axial positions of Homer1, PSD-95 and Bassoon along the trans-synaptic axis (defined as the axis through the center of the DP pattern and the PSD center). In agreement with previous work, Homer1 is on average localized further inside the spine and away from the cleft than the PSD center, while PSD-95 is concentrated directly onto the PSD33. The measured distance between Bassoon and Homer1 of ~90–120 nm is consistent with previous reports using super-resolution imaging (compare with 70–127 nm, ref. 29 or 90–155 nm, ref. 31). Consistent with previous findings, synapses with thin or absent protein-dense PSDs were colocalized with Bassoon scaffold protein (Fig. 3q–s), but not PSD proteins Homer1 (Figs. 3jj–ll) and PSD-95 (Figs. 3ccc–eee), suggesting that they are inhibitory synapses34.
a, NHS ester (grayscale) pan-stained and Bassoon (red) immunolabeled brain tissue section. b, Magnified area in the red box in a showing a synapse. c, Bassoon immunolabeling of the same area as in b. d, NHS ester pan staining of the same area as in b. e–p, Further examples similar to b–d showing lateral (e–m) and top (n–p) views of Bassoon-immunolabeled synapses. q–s, Lateral view of a type II synapse pan-stained with NHS ester and immunolabeled with Bassoon antibody indicating that type II synapses are positive for Bassoon protein. t, NHS ester (grayscale) pan-stained and Homer1 (magenta) immunolabeled brain tissue section. u, Magnified area in the magenta box in t showing a synapse. v, Homer1 immunolabeling of the same area as in u. w, NHS ester pan staining of the same area as in u. x–ii, Further examples similar to u–w showing lateral (x–ff) and top (gg–ii) views of Homer1-immunolabeled synapses. jj–ll, Lateral view of a type II synapse pan-stained with NHS ester and immunolabeled with Homer1 antibody indicating that type II synapses are negative for Homer1 protein. mm, NHS ester (grayscale) pan-stained and PSD-95 (cyan) immunolabeled brain tissue section. nn, Magnified area in the cyan box in mm showing a synapse. oo, PSD-95 immunolabeling of the same area as in nn. pp, NHS ester pan staining of the same area as in nn. qq–bbb, Further examples similar to nn–pp showing lateral (qq–yy) and top (zz–bbb) views of PSD-95-immunolabeled synapses. ccc–eee, Lateral view of a type II synapse pan-stained with NHS ester and immunolabeled with PSD-95 antibody indicating that type II synapses are negative for PSD-95 protein. fff, NHS ester (grayscale) pan-stained and Synaptophysin (SYN; yellow) immunolabeled brain tissue section. ggg, Magnified area in the yellow box in fff showing a synapse. hhh, Synaptophysin immunolabeling of the same area as in ggg. iii, NHS ester pan staining of the same area as in ggg. jjj–lll, Further example similar to ggg–iii. mmm, Comparison between Bassoon–PSD and DP–PSD distances (n = 74 measurements from 3 FOVs in 1 independent experiment, Welch’s two-sided t-test, P = 1.13 × 10−6). nnn, Comparison between Homer1–PSD and DP–PSD distances (n = 120 measurements from 5 FOVs in 2 independent experiments, Welch’s two-sided t-test, P = 1.64 × 10−14). ooo, Comparison between PSD-95–DP and DP–PSD distances (n = 113 measurements from 3 FOVs in 1 independent experiment, Welch’s two-sided t-test, P = 3.00 × 10−10). ****P < 0.0001. ppp, Relative spatial distributions of Bassoon, PSD-95 and Homer1 along the trans-synaptic axis. Medians and interquartile ranges are shown with whiskers drawn down to the minimum and maximum values. Panels showing pan staining are displayed with a white-to-black color table; panels showing immunolabeling are displayed with a black-to-white color table. All images (with the exception of fff–lll) are z-projections (intensity average) of two images. All NHS ester images were Gamma-corrected with γ = 0.7 with the exception of the Synaptophysin images (γ = 0.6). All scale bars are corrected for the expansion factor. Scale bars: 2 µm (a,t,mm), 200 nm (b–m,q–s,u–ff,jj–ll,nn–yy,ccc–eee,ggg–lll), 100 nm (n–p,gg–ii,zz–bbb), 1 µm (fff). Expansion factor: 21.3 ± 1.5.
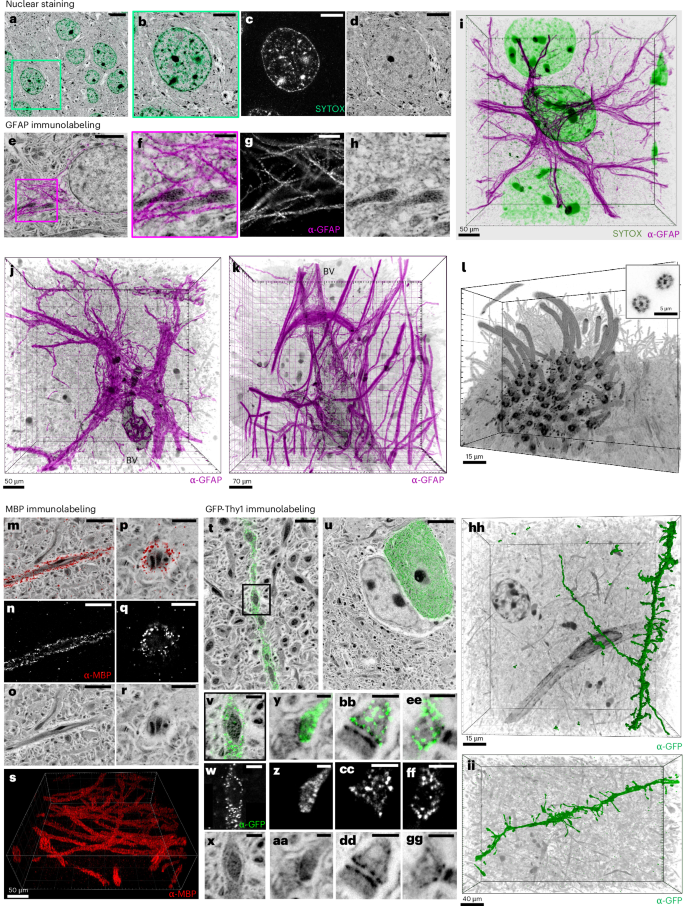
pan-ExM-t is not limited to providing context to synaptic proteins. We found that SYTOX Green produces a bright nuclear staining that overlays perfectly with NHS ester pan-stained cell nuclei (Fig. 4a–d). Furthermore, we can resolve individual filaments of glial fibrillary acidic protein (GFAP) in astrocytes (Fig. 4e–k) including near multiciliated ependymal epithelia (Supplementary Fig. 18) and engulfing blood vessels (Fig. 4k and Supplementary Video 7). The distinctive pan-stain pattern of astrocyte nuclei and their thick and fibrous cytoplasmic branches allow for their identification without GFAP, analogous to heavy-metal stains in EM40,41 (Supplementary Fig. 21 and Supplementary Video 8). We also observe that structures enriched in myelin basic protein (MBP) show lighter pan staining, suggesting these represent myelinated axons (Fig. 4m–s). Moreover, when we immunolabeled GFP in Thy1-GFP transgenic mice, we noticed the sparsity of this marker in both neuropil and neuron somas (Fig. 4t–ii, Extended Data Fig. 2 and Supplementary Video 9). Originally designed to resolve individual dendrites and axons in densely packed neuropil42, GFP-Thy1 imaged with pan-ExM-t reveals these transgenic neurons in their ultrastructural context. pan-ExM-t clearly shows the GFP surrounding, but being excluded from, mitochondria (Fig. 4v–x). The ability to distinguish GFP-positive dendritic spine heads (Fig. 4y–aa) and axonal boutons (Fig. 4bb–gg) from their synaptic partners suggests that pan-ExM-t is well-suited for light-based neural tracing and connectomics.
a, Cortical neurons pan-stained with NHS ester (grayscale) and stained with SYTOX Green (teal) showing a neuron nucleus. b–d, Magnified view of the area in the green box in a (b) and the SYTOX Green (c) and NHS ester (d) channels shown separately. e, Astrocyte pan-stained with NHS ester (grayscale) and immunolabeled with GFAP antibody (α-GFAP; magenta). f–h, Magnified view of the area in the magenta box in e (f) showing GFAP filaments surrounding mitochondria, and the α-GFAP (g) and NHS ester (h) channels shown separately. i, 3D rendering of α-GFAP (magenta), SYTOX Green (green) and NHS ester (grayscale). j,k, Two example 3D renderings of α-GFAP (magenta) and blood vessels (BV) pan-stained with NHS ester (grayscale). l, Multiciliated ependymal epithelia pan-stained with NHS ester (grayscale). Inset: ninefold symmetry of cilia. m–r, Lateral (m–o) and top (p–r) views of axons pan-stained with NHS ester (grayscale) and immunolabeled with MBP antibody (α-MBP; red). α-MBP (n,q) and NHS ester (o,r) channels of the areas shown in m and p. s, 3D rendering of anti-MBP showing multiple axons. t,u, Neuropil pan-stained with NHS ester (grayscale) and anti-GFP (green) showing that only a sparse subset of neurites (t) and cell bodies (u) express GFP-Thy1. v–x, Magnified area in the black box in t (v) showing a neurite pan-stained with NHS ester (x) and immunolabeled with anti-GFP (w) where GFP-Thy1 is not expressed inside mitochondria. y–gg, A dendritic spine (y–aa) and axonal boutons (bb–gg) pan-stained with NHS ester and immunolabeled with anti-GFP. z,cc,ff, Anti-GFP channels of the areas shown in y, bb and ee, respectively. aa,dd,gg, NHS ester channels of the areas shown in y, bb and ee, respectively. hh, 3D representation of a surface-rendered dendrite immunolabeled with anti-GFP (green) and NHS ester pan staining (grayscale). ii, 3D rendering of a dendrite immunolabeled with anti-GFP (green) and NHS ester pan staining (grayscale). Panels showing pan staining are displayed with a white-to-black color table; panels showing immunolabeling or SYTOX Green staining are displayed with a black-to-white color table. Gamma corrections: a,e,m,p,t,u,x,aa,dd,gg, γ = 0.7. 3D renderings were processed with Imaris with Gamma corrections applied. 3D image processing details for images i–l, s, hh and ii are found in the Methods section. Scale bars in the 3D renderings i–l, s, hh and ii are not corrected for the expansion factor. All other scale bars are corrected for the expansion factor. Scale bars: 5 µm (a), 2 µm (b–d,e,m–o,u), 500 nm (f–h,v–x), 1 µm (p–r,t), 200 nm (y–gg). Expansion factor: 22 ± 3.
Synaptic circuit tracing with pan-ExM-t
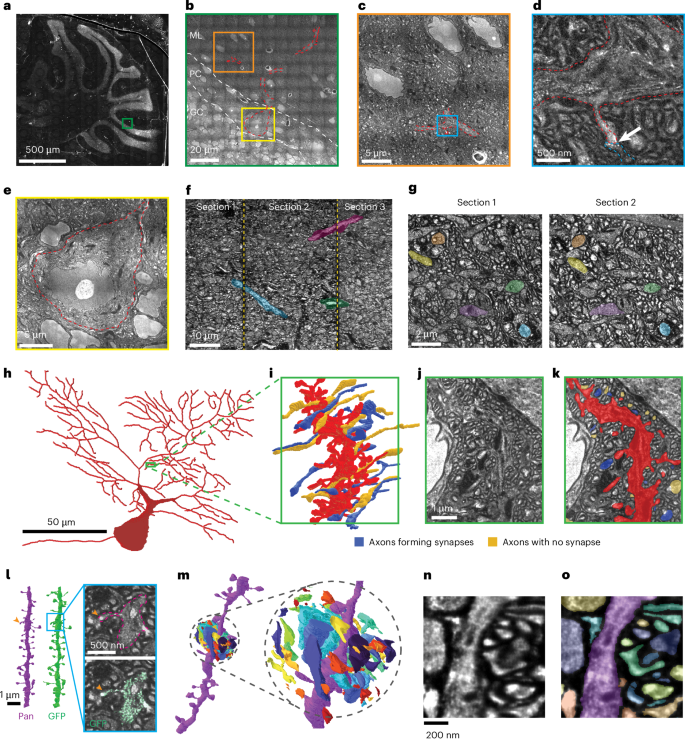
Mapping the fine morphology and connectivity of 3D neuronal networks typically requires specialized EM systems43, but pan-ExM-t makes it possible to achieve this using conventional light microscopy. To assess the feasibility of using pan-ExM-t to trace neuronal wiring diagrams (connectomes), we acquired large-scale datasets of tiled pan-ExM-t image volumes and evaluated circuit tracing based on these data. We found that tissue sections encompassing entire mouse brain sections could be expanded and labeled, and that regions containing circuits of interest could be rapidly imaged using spinning disk confocal microscopy (Fig. 5a–e and Extended Data Fig. 3). For example, we imaged a 2.2 × 2.2 × 0.14-mm (~125 × 128 × 8 µm pre-expansion) large circuit volume at 0.1 × 0.1 × 0.5 µm voxel size (~6 × 6 × 28 nm pre-expansion) in less than 5 hours (total volume size of ~250 gigavoxels, Methods). For a given section, the imageable volume is limited in the Z direction by the working distance of the objective. However, we demonstrate that this limitation can be overcome by sectioning the expanded tissue using a tissue slicer (Methods). Serial sections can be imaged and aligned back together to form a continuous 3D volume, enabling tracing of neuronal wiring across the interfaces (Fig. 5f,g).
a–e, Progressively zoomed-in images of a large pan-ExM-t sample from mouse cerebellum. a, Phase contrast, wide-field image of expanded sample encompassing the entirety of the cerebellum within a brain slice. b, High-resolution (spinning disk confocal) montage of a subvolume (indicated in a, green) spanning granule cell (GC), Purkinje cell (PC) and molecular layers (MLs). Portions of a Purkinje cell visible in this plane are indicated in red outlines. c, Zoomed-in field of view corresponding to subvolume indicated in b (orange). d, Further zoom-in view corresponding to subvolume indicated in c (cyan). Arrow indicates a synapse between a granule cell (parallel fiber) axon and a Purkinje cell spine. e, Zoom-in view of Purkinje cell body also shown in b,h. f,g, Demonstration of serial sectioning approach for large-volume imaging. f, Side view of three serial sections imaged and aligned. Large features (myelinated axons and blood vessels) crossing the cut boundaries are highlighted in color. g, Plan view of aligned surfaces from adjacent sections. Individual neuronal processes can be matched across both images, with several examples highlighted in color. h, Visualization of a Purkinje cell morphology whose processes have been traced throughout the entirety of the pan-ExM-t volume (also shown in b–e in red). i, 3D reconstruction of a section of the Purkinje Cell dendrite, showing dense dendritic spines (red) that terminate in synapses from parallel fiber axons (colors). j, Section of original pan-ExM-t data intersecting the structures visualized in i. k, Segmentation of structures visualized in i superimposed on pan-ExM-t data shown in j. l, Comparison of dendritic spines reconstructed from the pan channel only (magenta, inset top), versus with the aid of a neuron-specific GFP marker (GFP, inset bottom). m, 3D visualization of dense neuronal morphologies from a pan-ExM-t volume in mouse hippocampus. Inset: exploded view of dense cellular fragments shown in m, where space between fragments has been added to aid visualization. n, Virtual section of pan-ExM-t data corresponding to dense segmentations shown in m. o, Dense segmentation of cellular structures visualized in m superimposed on pan-ExM-t data shown in n. Gamma corrections: a, γ = 1.2; b–e, γ = 0.8; all others γ = 1. Expansion factor 17.9 ± 1.0.
By annotating these large 3D image volumes, we show that the large-scale morphology of neurons (Fig. 5h), as well as detailed 3D morphology of neuronal branches (Fig. 5i–k) can be reconstructed based on pan-ExM-t images. The high effective resolution achieved by pan-ExM-t made it possible to distinguish true synaptic contacts from closely passing neurites. We found that pan-ExM-t data was amenable to circuit tracing in a variety of brain areas, including the cortex and hippocampus (Extended Data Fig. 3). Critically, the ~20-fold expansion of pan-ExM-t attains the high resolution needed to trace the smallest neuronal wires, such as spine necks and thin axons, and unambiguously identify individual synaptic connections between neurons. To quantify the resolution, we estimated the point-spread function from small cellular features labeled by GFP (Methods). These measurements placed an upper bound on imaging resolution of 290 ± 60 nm and 840 ± 180 nm in the lateral and axial directions, respectively, corresponding to 14 ± 3 nm lateral and 42 ± 9 nm axial resolution in 20-times expanded samples (Extended Data Fig. 5). To test whether this resolution in combination with the pan-staining contrast is sufficient for connectomic data, we traced spiny dendrites from the pan channel of pan-ExM-t image volumes and compared them to the ‘ground truth’ based on transgenic expression of GFP (Figs. 4ii,hh and 5l). Based on the pan channel only, we were able to successfully trace 77 ± 7% of spines (mean ± s.d. of recall, n = 4 datasets, 199 spines), suggesting that the high-resolution pan channel is sufficient to accurately trace most spine necks (Fig. 5l and Supplementary Fig. 34, Methods). A major advantage of the pan-stain is that it labels all cells, rather than a sparse subset. To illustrate this distinction, we manually reconstructed all cellular structures within a small region of tissue (2 × 1 × 1 μm pre-expansion, Fig. 5m–o and Methods). We found that most (95%) of the reconstructed structures extended beyond the end of the bounding box, and that 40% of the tissue volume was excluded from cellular structures, which is consistent with our previously estimated ECS+ fraction of 38 ± 5% (Fig. 2j and Supplementary Fig. 13). This suggests that cellular morphologies can indeed be densely and accurately reconstructed from pan-ExM-t data.
pacSph enables lipid labeling of tissue with pan-ExM-t
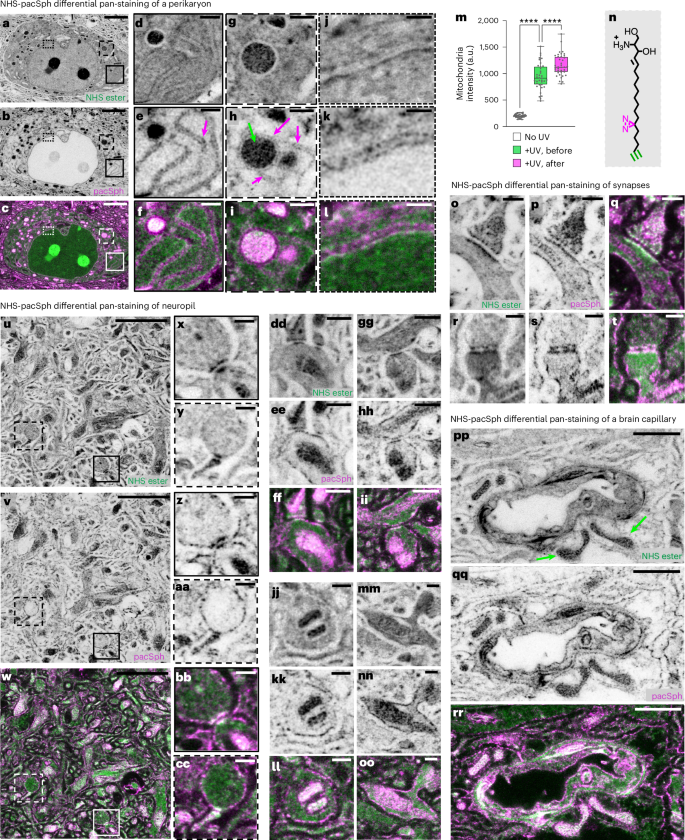
Neural connectomics analysis in EM generally relies on contrast from lipid membranes to delineate cellular boundaries. One way to achieve this in pan-ExM-t would be to preserve the lipid content. We therefore developed a new pan-ExM-t lipid labeling strategy where we use a commercially available photocrosslinkable and clickable sphingosine pacSph44 as a lipid-intercalating reagent (Fig. 6, Supplementary Figs. 30–32 and Methods). This strategy worked in our hands better than alternative approaches (Supplementary Methods). In the perikarya of brain tissue fixed with 4% formaldehyde + 20% AAm and pan-stained with pacSph, we can clearly discern both sides of endoplasmic reticulum tubules (Fig. 6a–f), endoplasmic reticulum and mitochondria contact sites (Fig. 6g–i), and both sides of the nuclear envelope (Fig. 6j–l). In neuropil, lipid membrane boundaries are now discernable (Fig. 6o–oo), revealing the boundaries of neurites and synaptic compartments. In brain capillaries, the different membranes are also differentially highlighted relative to NHS ester pan staining (Fig. 6pp–rr). Moreover, when we experimented with irradiating pacSph pan-stained tissue with ultraviolet (UV) light before or after the first hydrogel embedding, we found that UV irradiation after hydrogel embedding results in significantly higher probe retention, suggesting that diazirine is being photo-crosslinked to the dense hydrogel mesh in addition to surrounding proteins (Fig. 6m and Supplementary Fig. 31). In fact, a recent study showed that diazirine has an affinity for reacting with proteins containing large negative electrostatic surfaces in cells (for example, carboxyl groups)45. We suspect that diazirine in our probe is preferentially photo-crosslinked to carboxyl groups on the poly(acrylamide/acrylate) mesh, offering better retention of the lipid content. While benefiting from future investigation, our data show that photo-crosslinking lipid-intercalating ceramides to the expansion hydrogel represents a promising strategy for labeling lipids in ~20-fold expanded and formaldehyde fixed brain tissue sections.
a–c, Perikaryon pan-stained both with NHS ester (a) and pacSph (b) (overlay shown in c). d–l, Magnified areas of the solid boxes in a–c showing the two sides of endoplasmic reticulum tubules (magenta arrow; d–f), the dashed boxes in a–c showing a mitochondrion (green arrow) in contact with the endoplasmic reticulum (magenta arrow; g–i) and the dotted boxes in a–c showing the nuclear envelope (j–l). m, Mitochondria signal levels in tissue pan-stained with pacSph and not irradiated with UV (white; n = 35 measurements from 5 FOVs in 1 independent experiment); pan-stained with pacSph and irradiated with UV before hydrogel embedding (green; n = 37 measurements from 5 FOVs in 1 independent experiment) and pan-stained with pacSph and irradiated with UV after hydrogel embedding (pink; n = 39 measurements from 5 FOVs in 1 independent experiment). Statistical significance was assessed by Welch’s two-sided t-tests with Bonferroni correction (three comparisons): no UV versus +UV, before, P = 1.37 × 10⁻5; no UV versus +UV, after, P = 5.8 × 10⁻7; +UV, before versus +UV, after, P = 4.5 × 10⁻19). ****P < 0.0001. Medians and interquartile ranges are shown with whiskers drawn down to the minimum and maximum values. n, Chemical structure of pacSph. The probe is photo-crosslinked via its diazirine group (magenta) and labeled via its alkyne group (green). o–t, Synapses pan-stained both with NHS ester (o,r) and pacSph (p,s) (overlays shown in q,t). u–w, Neuropil pan-stained both with NHS ester (u) and pacSph (v) (overlay shown in w). x–cc, Magnified areas of the solid (x,z,bb) and dashed (y,aa,cc) boxes in u–w showing synapses. dd–oo, Neurites and synapses pan-stained both with NHS ester (dd,gg,jj,mm) and pacSph (ee,hh,kk,nn) (overlays shown in ff,ii,ll,oo). pp–rr, Brain capillary pan-stained both with NHS ester (pp) and pacSph (qq) (overlay shown in rr). Green arrows point to mitochondria. Panels showing individual color channels are displayed with a white-to-black color table. Gamma corrections: a, γ = 0.7; p,r,u,dd,gg,jj,mm,pp, γ = 0.5. All scale bars are corrected for the expansion factor. Scale bars:3 µm (a–c), 500 nm (d–i,dd–ii), (2 µm) u–w, 250 nm (j–l,o–t,x–cc,jj–oo),1 µm (pp–rr). Expansion factor 18.1 ± 1.3.