Minimum four families filed a lawsuit against the manufacturer of infant formula ByHeart claiming their children contracted botulism from contaminated formula milk as the company comes under continued scrutiny from federal investigators and a separate class-action lawsuit was filed last week.
In lawsuits, affected families described agonizing days or weeks spent in the hospital with their children, who were placed on IVs and feeding tubes. Many said they chose the ByHeart formula because it contains organic whole milk and minimal additives, making it the healthiest option.
The company stated in statement On Wednesday, lab tests revealed Clostridium botulinum spores in samples of his formula. ByHeart told NBC News that it could not comment on pending litigation and that “the company is currently focused on the recall and root cause investigation.”
According to the Food and Drug Administration, 31 babies in those who consumed the mixture, botulism is suspected or confirmed. The cases span 15 states and all have required hospitalization. No deaths were reported.
The bacteria that causes botulism can grow in foods that are not preserved or preserved properly and produce a toxin that attacks the nerves. The resulting illness can cause difficulty breathing, muscle paralysis, or death.
ByHeart said on its website that it had not determined the root cause of the contamination, but shared its test results with the FDA.
“We immediately notified the FDA of these findings and are working to investigate the facts, conduct ongoing testing to identify the source, and ensure this does not happen to families again,” it said.
In an interview with NBC News, Hannah Everett said she started giving ByHeart formula to her daughter Piper at about 2 months. By early this month, Piper became constipated, was drooling profusely and her left eye seemed droopy, Everett said. A friend sent her a link to a ByHeart review.
“Of course, the can she just finished that day had the exact lot number that was affected,” said Everett, who lives in Richmond, Kentucky.
On November 9, Piper was admitted to a children's hospital where she was diagnosed with botulism. Everett said the sight of doctors and nurses struggling to insert IVs and a feeding tube made her want to vomit.
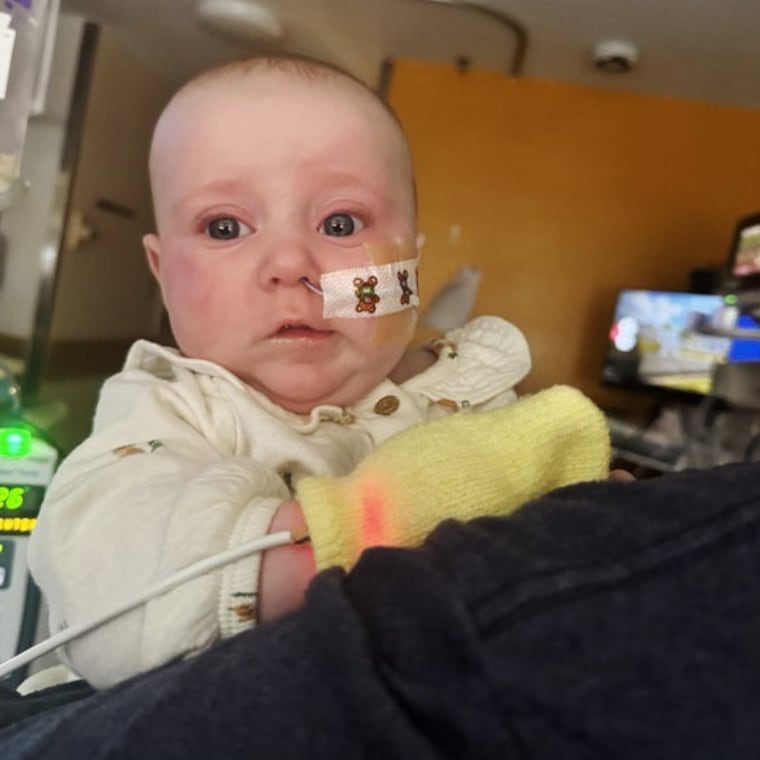
She said two friends had to physically support her “because I was just sobbing.”
“They're holding your baby, who technically wasn't even four months old at the time, and she's just screaming bloody murder. And there's nothing you can do,” Everett said.
Piper was given botulism antitoxin intravenously. Medicines are not always available in hospitals, so they had to be brought by plane. Everett said Piper's condition had improved; she was released from the hospital about a week ago.

But Everett is still wracked with guilt.
“I feel like I let her down when I know I didn't. It's hard to tell yourself that as a mother because you'll blame yourself,” she said.
Everett and her husband Michael sued ByHeart last week, seeking damages for medical expenses, pain and suffering.
“It makes me even more angry and just sick that it took them so long to admit it,” she said. “It's almost like too little, too late.”
Everett said she wrote to ByHeart about the review while Piper was in the hospital and they offered to send her more bottles of formula.
Darin Detwiler, a professor of food regulatory policy at Northeastern University, agrees that ByHeart should have taken comprehensive action sooner.
“They should have identified it themselves and reported it immediately,” he said.
After the FDA warned ByHeart about a potential link between its formula and the botulism outbreak, the company initially recalled only two lots. The next day, ByHeart posted on its website that there was not enough evidence to link its product to the diseases because the sample that tested positive for botulism bacteria came from an open jar that “can be contaminated in a variety of ways.”
In court documents, parents suing ByHeart describe states of horror.
In the latest lawsuit, filed Wednesday, the Washington state couple said their daughter has chronic constipation, difficulty feeding and extreme fatigue while taking formula. She was admitted to the emergency room at 2 months old, the document states.
The family left the hospital Wednesday, according to the lawsuit. The mother, Madison Wescott, said she wasn't producing enough milk to meet her daughter's needs without formula.
“Knowing that I could not adequately feed my child and could not trust the formula companies had a serious impact on our family,” Wescott said in the lawsuit.
In California, Anthony Barbera and Talia Flores fed their son exclusively ByHeart formula after his birth, according to their lawsuit. By the time their son received botulism antitoxin at the hospital, he was no longer eating, was hooked up to multiple IVs and was too weak to cry, their lawsuit says.
Arizona parents Steven and Yurani Dexter said in their lawsuit that their daughter stopped eating altogether in August, refusing a bottle of formula as soon as it touched her lips. She was taken by ambulance to a children's hospital. The couple said they feared she might die or never fully recover.
Bill Marler, a lawyer representing the Dexters, Wescotts, Barberas and Flores, said ByHeart “has a lot to answer for.”
“If there is one product that should be safe, it should be infant formula,” he said.
Before this, no botulism outbreak in the United States had been linked to infant formula. Formula manufacturers are not required to routinely test for Clostridium botulinum, but they must follow health controls to prevent contamination and are subject to FDA inspections.
Most of the major formula recalls in recent years, including the 2022 Abbott Nutrition recall that contributed to a national formula shortage, have been caused by potential contamination with another bacterium, Cronobacter sakazakii. ByHeart also recalled batches of its formula in December 2022 due to possible Cronobacter contamination.
In 2023 the FDA sent a warning letter ByHeart described “serious irregularities” at its Pennsylvania manufacturing facility. The FDA said ByHeart linked a batch of the formula to a positive Cronobacter test result due to a laboratory error, although the lab denies this. The agency also said the facility had two water leaks and that ByHeart did not evaluate the potential link between the leaks and the formula, which later tested positive for Cronobacter.
ByHeart's website says the company has “taken action to resolve the issues and there are no open issues in this warning letter.” The Pennsylvania plant was not involved in formula production in the current recall, the company said.
Abigail Snyder, an assistant professor of microbial food safety at Cornell University, said an FDA warning letter like the one ByHeart received is “quite unusual,” although regulatory activity on baby food has increased since the Abbott recall.
“Unfortunately, fewer ingredients and whole milk are a different attribute than microbial safety,” she said.








