Magnified view of a brain organoid with fluorescent labeling of various cell types.Photo: Thomas Hartung, Johns Hopkins University.
Advanced tools and techniques give researchers a detailed look at the body's defense system. The methods described below will help immunologists expand the scope of their work in 2025, from detecting single cells in action to improving or replacing animal models.
THX mice: mouse on the outside, human on the inside
Since the first mouse models with human cells and tissues were developed more than 40 years ago, they have become much better at mimicking the human immune system. But they are still not accurate enough.
One of the main problems is that mice have more than 1,600 immune response genes that do not match their human equivalents. And most “humanized” mice (those with human genes, cells or tissue) don't produce enough antibodies to fight infections, which is a problem for researchers studying vaccines and immune responses, says Paolo Casali, an immunologist at the University of Texas Health Science Center at San Antonio. “They are completely useless,” he says.
Nature Index 2025 Immunology
The team, led by Casali and his former graduate student Daniel Chappe, now an immunologist at the Massachusetts-based biotech company Invivyd, has been trying to overcome this hurdle for decades, and their efforts are starting to bear fruit. In an article published last year1the team introduced THX, a mouse model that replicates the human immune system.
To create THX, mice are injected with human stem cells, which develop into immune components such as lymph nodes, antibodies, and T and B cells (types of white blood cells). When they were given the mRNA COVID-19 vaccine, they had a strong immune response—an important trait for vaccine research.
THX mice have attracted the attention of several research groups in the US and Europe. “I get emails almost every day,” Casali says. Among these groups is one led by James Voss, an immunologist at Scripps Research in La Jolla, California, who plans to use mice to advance his work on targeted therapies for HIV. Voss and his team used CRISPR gene editing to reprogram human B cells to produce antibodies that can recognize and block many different strains of HIV. And although existing humanized mouse models contain B cells, they fail to produce a strong enough antibody response, creating what Voss calls a “developmental block.” THX mice overcome this limitation, opening a new avenue for his research.
Once on the market, THX mice are expected to be relatively cheap and less labor-intensive to manufacture than other mouse models. That's because they don't require complex tissue transplants, says Voss, who hopes to start using them in the coming months. “I'm very excited. I think it's going to be a game changer.”
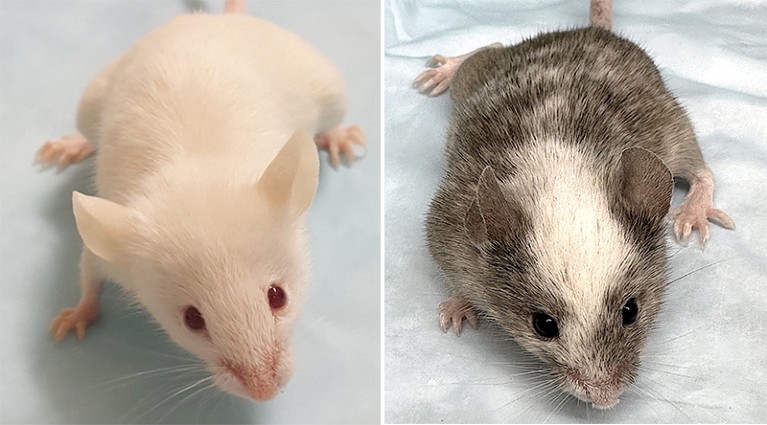
Various versions of the THX mouse model replicating the human immune system.Photo: Daniel Chapp (left); Carlos E. Rivera (right)
Organ-on-a-chip and organoid systems: move over, mice
Some researchers rejection of animal models basically by creating miniature versions of human organs and tissues. These systems, which include organ-on-a-chip platforms and organoid systems (cell cultures that mimic organ function) provide images of a person's intestines, liver, lungs and other organs in both disease and health.
“This is an attractive prospect for drug development because these systems can provide a more accurate model of human biology than animal models,” says Thomas Hartung, a pharmacologist at Johns Hopkins University in Baltimore, Maryland. He adds that many of the drugs approved over the past decade are based on human proteins and antibodies. “There is no point in testing them in animal models.”
Models of tiny organs also help researchers better understand how the body's systems interact with each other and respond to disease. Linda Griffith, a bioengineer at the Massachusetts Institute of Technology in Cambridge, and her colleagues developed a model of the human gut to study how the organ's microbes interact with immune cells and regulate inflammation. They also created models of endometriosis and pancreatic cancer.
Creating such systems and trying to replicate them is difficult, and many labs are unable to do so, Griffith said. She adds that scaling them up for mass production is also very difficult because it requires intensive quality control measures.
Despite these obstacles, organ and tissue microsystems are likely to become more common in laboratories, at least in the United States. In July, the US National Institutes of Health (NIH) announced that it would no longer offer new funding projects that rely solely on animal models to study human diseases. Instead, the NIH will prioritize research that uses human technologies, including organ-on-a-chip and organoid systems.
US Food and Drug Administration (FDA) announced similar plans this year phase out animal testing in favor of human cell cultures and organoids for drug safety testing. The FDA roadmap notes that animal models cannot predict the success of drugs for many common diseases, from cancer to Alzheimer's disease. Some compounds that appeared safe in animal models have proven lethal in humans, according to the regulator. “They tell us very clearly what the future looks like,” Hartung says.
ScRNA-seq: Advancing Single Cell Sequencing
Few techniques have helped researchers unravel the mysteries of the immune system, such as single-cell RNA sequencing (scRNA-seq), a method of determining which genes are transcribed into the RNA of individual cells. Introduced in the late 2000s, scRNA-seq has developed rapidly over the past decade and has been used to map specific immune cell types and identify key differences in gene activity that influence immune response. Now researchers are pairing scRNA-seq with other tools to give it other capabilities.
One method that pairs particularly well with scRNA-seq is CITE-seq, which simultaneously identifies both RNA and proteins on the surface of individual cells, says Sarah Suliman, an immunologist at the University of California, San Francisco.
This combination approach has been a boon for research into natural killer (NK) cells (white blood cells that destroy diseased cells), says Eric Vivier, an immunologist at the Center for Immunology of Marseille-Luminy in France. Last year, Vivier's team published a study2 who used scRNA-seq and CITE-seq to identify three major types of NK cells based on the proteins they produce in different tissues and cancer cells. This has helped researchers develop more effective cell “invaders”—molecules that direct NK cells to attack tumors. The team set out to create a new activator that activates all three types of NK cells to fight treatment-resistant forms of non-Hodgkin's lymphoma.3.
Such efforts would not be possible using older technologies, such as bulk RNA sequencing, which obscures the distinct signatures of individual cells, Vivier said.
Another extension of scRNA-seq, called single-cell ATAC-seq, highlights which regions of DNA are open, accessible, and potentially active in regulating gene expression, rather than simply showing which genes are turned on. This method not only provides insight into cellular function, but also allows researchers to piece together what is possible or what might happen in the future. In August, a team of researchers from Tsinghua University School of Medicine in Beijing described how they used ATAC-seq to study acute myeloid leukemia, a rapidly progressing cancer of the blood and bone marrow.4. The work showed how dysregulation of genes in some leukemia cells can lead to a return of the disease after treatment.
Meanwhile, Perturb-seq is a method that combines scRNA-seq with CRISPR gene editing. This allows scientists to turn off or modify genes in thousands of cells at once and monitor how those changes affect the cells. It has been used to study cause-and-effect relationships, such as how a small genetic change can make a person more vulnerable to infection or inflammation, Suliman said. “That’s where the powerful data is.”